Managing DED with lipid and non-lipid eye drops
The mainstay therapy for dry eye disease (DED) is the use of artificial tear solutions to supplement the natural tear film1. The therapeutic landscape has recently seen a considerable evolution of topical eye drops. Formulations often incorporate lipid components to address tear lipid deficiency common in meibomian gland dysfunction and the associated evaporative, predominant subtype of DED2. Practitioners seeking guidance in their choice of artificial tear supplements for the treatment of dry eye disease face a dearth of sound scientific evidence. Few studies compare the efficacy of lipid and non-lipid formulations across the breadth of dry eye subtypes and the quality of evidence is generally considered to be poor1-7.
Most studies in this area focus on short-term effects of drop instillation, often with one month or less of use7. Little is known about the time course of clinical improvements of DED signs and symptoms and whether treatment effects differ according to disease subtype. Longer-term efficacy studies that more closely resemble intended clinical use can inform clinicians and patients about the recommended length of treatment regimes. By setting realistic expectations on the time course of clinically significant improvements in symptoms, an evidence-based approach may assist practitioners in encouraging patient compliance.
The aim of this study was to evaluate the therapeutic efficacy of a lipid- and a non-lipid-based artificial tear supplement for the management of DED in a six-month study. Striving to achieve a superior level of evidence quality, a double-blind, randomised trial study design was chosen, and the study conducted across study sites in New Zealand, Canada, the UK and Australia. Dry eye was diagnosed using the TFOS DEWS II global consensus criteria9.
Study design
Ninety-nine participants (64% female, mean age 44 ± 16 years, between 21 and 75 years) with manifest symptoms and signs of DED were recruited. Participants were randomised to a four times daily, bilateral administration of either an aqueous-based drop or a combination lipid-aqueous nanoemulsion (Systane Ultra and Systane Complete, Alcon, USA), dispensed in masked bottles. Study outcomes were evaluated on a monthly basis following baseline, for a total of six months.
Compliance was determined by returned bottle weight at each visit. Clinical tests and symptomology questionnaires were administered in accordance with the recommendations of the TFOS DEWS II Diagnostic Methodology subcommittee, in increasing order of invasiveness9.
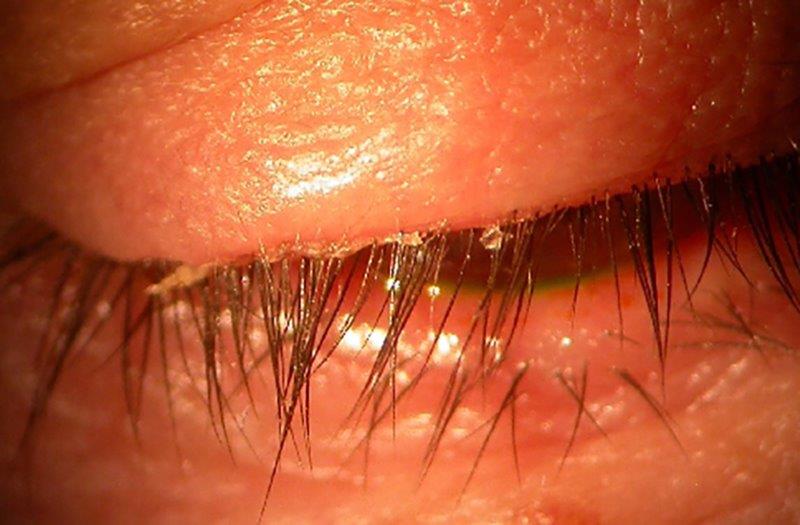
Blinking frequency and completeness, bulbar conjunctival hyperaemia, tear meniscus height, NIBUT, lipid layer grade (LLG) and upper and lower meibography were assessed using the Keratograph 5M (Oculus Optikgeräte, Germany). Tear film osmolarity was measured with the TearLab osmometer (Escondido, USA). An anterior segment inspection involving lid margins, eyelashes, meibomian gland expressibility and corneal and conjunctival staining was conducted using a slit-lamp biomicroscope. All measures were graded according to the TFOS DEWS II Diagnostic Methodology consensus criteria9.
Results
Across the four study sites, participants were comparable in terms of demographic and clinical baseline data (p>0.05). The returned eye drop bottle weight suggested good product administration compliance, indicating the application of approximately four drops daily.
At baseline, all participants fulfilled the TFOS DEWS II criteria for DED. By the end of the study, one in five participants (19%) no longer had dry eye disease.
Fig 1. Recovery of DED status in the lipid and non-lipid groups at each study visit
Results demonstrated sustained reductions in both treatment groups in terms of dry eye symptomology (for OSDI, DEQ-5 and SANDE questionnaire scores), tear film stability, superior lid wiper epitheliopathy (LWE) grade, sodium fluorescein and lissamine green staining scores (all p<0.05). Interestingly, these measures of improvement were identified at different timepoints, showing sustained effects thereafter: symptomology from month one onwards (all p≤0.01); LWE from month two onwards (all p≤0.01); and tear film stability and corneal and conjunctival staining from month four onwards (all p<0.05). The improvements in tear film lipid layer quality were limited to the lipid-based tear supplement group and occurred from month three onwards (all p<0.05).
There were no significant changes across the study period or differences between study groups pertaining to tear meniscus height and tear osmolarity (all p>0.30); conjunctival hyperaemia, inferior LWE, eyelid margin and eyelash characteristics, meibomian gland dropout and meibum expressibility and quality (all p>0.05); and blink rate and the proportion of incomplete blinks (all p>0.10).
A positive response to treatment was defined as an improvement from baseline exceeding 4s in NIBUT and/or 4.5 points on the OSDI scale within an individual participant10,11. After one month of treatment, 70% of participants responded to treatment, with no difference between study groups. By six months, this proportion exceeded 74% of participants. The average improvement of these ‘responders’ throughout the study period was 4.5 ± 5.6 seconds in NIBUT and 16.6 ± 12.8 in OSDI score. Non-responders, however, registered an overall worsening of -2.4 ± 10.5 in OSDI and of -0.5 ± 3.7 seconds in NIBUT.
Table 1: Comparisons with baseline measurements at each time point within treatment groups. Data are presented as p-values. Green highlighting denotes statistically significant differences (p<0.05).
Conclusions
The results of this study highlight the importance of robust, high quality study design to elucidate the more subtle, medium-to-long term effects of artificial tear solution use for tear film and ocular surface improvement. The double-masked, randomised, multicentre design ensured a high degree of bias reduction, while the six-month study duration, backed by reliable measures of participant compliance to treatment, ranks this among the most extensive studies of its kind.
The two formulations demonstrated similar performance, with mild-to-moderate aqueous deficient and evaporative cases responding well to each. However, predominantly evaporative dry eye disease forms (lipid layer grade ≤ 3 in this study) may benefit most from lipid-based supplementation. It is noted that this study cohort did not include moderate-to-severe cases.
Lipid- and non-lipid-based artificial tear solutions offered rapid symptomatic relief within one month of regular, daily use, while more profound, structural improvements in tear film and ocular surface integrity were observed only after several months of drop use. Notably, these results suggest that artificial tear solutions may have a therapeutic, rather than solely palliative effect if used consistently and regularly. Clinicians are therefore encouraged to endorse regular drop use to improve tear film and ocular surface health and the progression of dry eye disease.
Tear lipid layer grade improvements were limited to the group applying the lipid-based drop, from three months onward, while tear film stability improved significantly and sustainedly (for both formulations) from month four. The underlying mechanism is unclear but more than a simple transient effect may be at play. Improvements in superior LWE suggest that daily, sustained, repeated lipid supplementation can affect the ocular surface physiology and improve blinking-related friction. This might reasonably be expected to contribute to restoring tear film homeostasis, breaking the vicious cycle of dry eye disease and promoting improved meibomian gland function and ocular surface health.
Nearly one in five participants no longer met the classification criteria for DED, according to TFOS DEWS II, following six months of drop use9. Interestingly, determining whether a patient would respond to treatment or not appears to be a decision that can be made after one month of use, as those who did not respond favourably in this time frame did not subsequently show significant improvement symptomatically.
Of note, around 30% of participants did not respond to therapy; in the clinical setting this would indicate that it is appropriate to explore alternative and/or supplementary management strategies after a one-month trial. For patients who present with a positive symptomatic improvement after one month (evaluated using validated questionnaires), continuation of therapy may be beneficial. The gradual and relatively late improvements in clinical signs (compared to symptomatic improvements) observed in this study indicate that restoring ocular surface homeostasis hinges on the prolonged, regular tear film supplementation over several months. As always, compliance is paramount.
Acknowledgements
We would like to acknowledge our colleagues in New Zealand: Dr Michael Wang, Dr Ally Xue, Sophie Speakman, Cameron Dean; in Canada: Dr Doerte Luensmann, Dr Alison Ng, Dr Hendrik Walther, Dr Marc Schulze, Dr Jill Woods, Professor Lyndon Jones; in the UK: Sònia Travé Huarte, Dr Alec Kingsnorth, Professor James Wolffsohn; and in Australia: Dr Jacqueline Tan, Dr Jaya Siddireddy, Dr Katherine Wong, Professor Fiona Stapleton, Professor Mark Willcox. Financial support was received for this investigator-initiated trial from Alcon Laboratories (Australia) Pty Ltd. The funding source had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation of the manuscript; or the decision to submit the manuscript for publication.
References
- Jones L, Downie LE, Korb D, Benitez-del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II Management and Therapy Report. Ocul Surf 2017;15:575–628.
- Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf 2017;15:276–83.
- Simmons PA, Carlisle-Wilcox C, Vehige JG. Comparison of novel lipid-based eye drops with aqueous eye drops for dry eye: a multicenter, randomized controlled trial. Clin Ophthalmol 2015;9:657–64.
- McCann LC, Tomlinson A, Pearce EI, Papa V. Effectiveness of artificial tears in the management of evaporative dry eye. Cornea 2012;31:1–5.
- Tomlinson A, Madden LC, Simmons PA. Effectiveness of dry eye therapy under conditions of environmental stress. Curr Eye Res 2013;38:229–36. doi:10.3109/02713683.2012.757323.
- Guthrie SE, Jones L, Blackie CA, Korb DR. A comparative study between an oil-in-water emulsion and nonlipid eye drops used for rewetting contact lenses. Eye Contact Lens 2015;41:373–7.
- van der Westhuizen L, Pucker AD. Over the counter (OTC) artificial tear drops for dry eye syndrome: A Cochrane review summary. Int J Nurs Stud 2017;71:153–4.
- Bruix A, Adán A, Casaroli-Marano RP. Efficacy of sodium carboxymethylcellulose in the treatment of dry eye syndrome. Arch Soc Esp Oftalmol 2006;81:85–92.
- Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf 2017;15:539–74.
- Miller KL, Walt JG, Mink DR, Satram-Hoang S, Wilson SE, Perry HD, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol 2010;128:94–101.
- Wang MTM, Ganesalingam K, Loh CS, Alberquerque T, Al-Kanani S, Misra SL, et al. Compatibility of phospholipid liposomal spray with silicone hydrogel contact lens wear. Contact Lens Anterior Eye 2017;40:53–8.


Optometrist Dr Alex Müntz is a post-doctoral research fellow working in the Ocular Surface Laboratory at the University of Auckland, which is headed by Professor Jennifer Craig.