New hope for DR sufferers
Professor Colin Green was invited to deliver a presentation at the prestigious Association for Research in Vision and Ophthalmology (ARVO) annual meeting in Vancouver on his team’s research into diabetic retinopathy as part of their ongoing hunt for better treatments.
Using a unique model of diabetic retinopathy (DR) developed at Auckland University, the team’s research modulates cell membrane channels called connexin hemichannels to suppress inflammation and recover function.
These connexin hemichannels play an important role in wound-healing and chronic inflammatory diseases, said Prof Green. “The inflammasome pathway, which is part of our innate immune system, is activated in just about every chronic inflammatory disease, including macular degeneration and diabetic retinopathy.”
Our body’s immune response can be triggered by many factors, such as genetic predisposition, traumatic brain injury, infection, diabetes or even just inflammatory cytokines circulating in the bloodstream following an accident or stress. These signals initiate the disease process, priming the inflammasome pathway, but hemichannel opening then releases ATP (adenosine triphosphate) which acts as a second activating signal, both amplifying and perpetuating the inflammation, explained Prof Green.
“As we get older, we lose the ability to shut the inflammation down, which is probably why so many of these diseases are classically age-related.”
In his ARVO presentation at the end of April, Prof Green focused on DR. “While diabetic retinopathy is related to diabetes, high glucose in the bloodstream on its own is probably not the problem. It’s the high glucose in combination with inflammatory cytokines.”
Following years of research, Prof Green’s team has developed three ways to regulate the connexin hemichannels, providing the basis for three potential new treatments to regulate the body’s inflammatory response and thus affect the deterioration of sight through non-healing corneal defects, DR, diabetic macular oedema and geographic atrophy (advanced dry AMD).
The first two connexin hemichannel blocker treatments being developed are Nexagon, an antisense oligonucleotide in final clinical trials, and a mimetic peptide (still at preclinical stage). The third potential drug treatment, based on the same principles, is Xiflam (tonabersat), an old SmithKline Beecham molecule that never went past Phase II. “But we looked at it again and realised it was acting as a hemichannel blocker,” said Prof Green, adding, better still, it can be taken as a tablet.
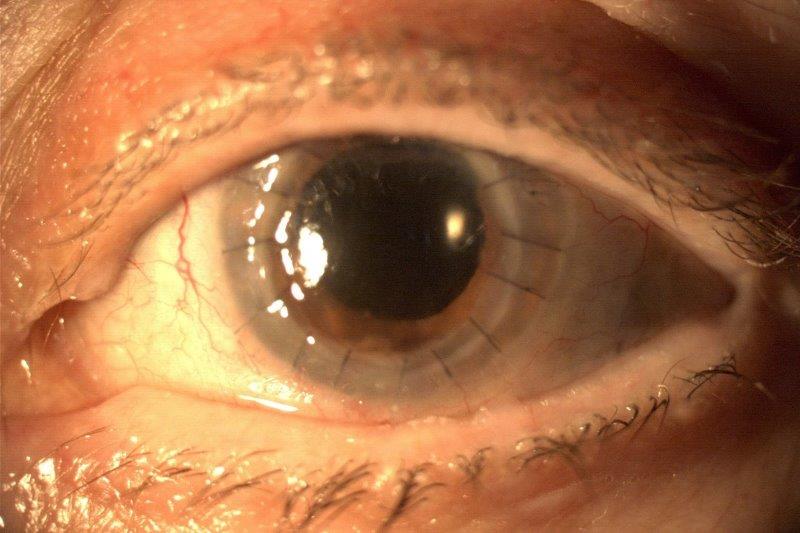
After re-patenting tonabersat, the research team put it into their DR model to treat lesions, vascular leakage and reduced retinal function. The rats were given oral tonabersat daily for two weeks and re-examined. Researchers found fewer lesions and full retinal function recovery. “That does not mean in someone who may have had DR for a long time that we are going to recover their vision completely,” said Prof Green. “But if neurons and receptors were not functioning optimally because of this inflammatory pathway, a high degree of vision recovery may be possible and, at the very least, disease progression ameliorated.
“The results clearly support the concept of a hemichannel blocker as a therapeutic for retinal diseases. To have two drugs coming from one department, at one university, that have got this far in clinical trials is a pretty good achievement and I think it all comes down to the fantastic team we’ve got.”
The team on the diabetic project comprises: Drs Monica Acosta, Lola Mugisho and Nasir Mat Nor (who were PhD students on the project), A/Prof Ilva Rupenthal and Prof Colin Green, working with ophthalmologists Prof Helen Danesh-Meyer and Dr David Squirrell.
Prof Green is named on more than 260 patent applications and received the University of Auckland Vice-Chancellor’s Commercialisation Medal in 2014. He is co-founder of Ocunexus Therapeutics in the USA, which is developing Nexagon and Xiflam. In October last year, Eyevance Pharmaceuticals acquired the worldwide rights to Nexagon, which will enter final clinical trials (run by OcuNexus) for non-healing corneal defects during the second half of this year.
Xiflam is scheduled to go straight to Phase IIB trials before the end of this year.