Non-IOP factors in glaucoma management
Current glaucoma treatment focuses on the reduction of intraocular pressure, via medical management, laser trabeculoplasty or surgery. However, a significant number of patients with primary open angle glaucoma (POAG), the most common type of glaucoma in New Zealand, will progress to visual impairment despite treatment. As such, it is important to identify non-IOP factors in glaucoma management. This brief review will focus on neuroprotection and ocular perfusion pressure in glaucoma, as these factors may be taken into account by ophthalmologists and optometrists managing these patients.
Neuroprotection in glaucoma
Neuroprotection is a direct therapeutic approach to prevent retinal irreversible ganglion cell damage or enhance cell survival. Topical and oral neuroprotective agents have been widely investigated.
Brimonidine
Brimonidine is an 2-adrenergic agonist, reducing the production of aqueous humour, and also enhancing uveoscleral outflow. In rat optic nerve injury models, brimonidine has been found to reduce the rate of retinal ganglion cell loss, independent of its IOP lowering effect. The postulated neuroprotective mechanisms include up-regulation of brain-derived neurotrophic factor in retinal ganglion cells, activation of anti-apoptotic genes and cell-survival signalling pathways, and modulation of NMDA receptor function. There has been one randomised, double-masked, multicentre clinical trial of brimonidine in glaucoma patients with untreated IOP of 21 mmHg. A total of 99 patients were randomised to receive brimonidine 0.2% eye drops twice a day, and 79 patients received timolol 0.5% twice a day. Over a mean follow-up period of 30 months, fewer patients in the brimonidine group had visual field progression compared with those treated with timolol (9.1% vs. 39.2%, p = 0.001). This was despite the fact that the mean treated IOP was similar between groups. It should be noted that a significantly higher number of patients dropped out of the brimonidine group, primarily due to localised ocular allergy.
A prospective (unmasked) study of 38 patients with POAG showed a small but statistically significant reduction in retinal nerve fibre layer (RNFL) thinning, with brimonidine 0.2% twice a day compared with timolol 0.5% once daily. Both groups, followed for one year, had similar IOP lowering from baseline. The mean reduction in average and quadrant RNFL thickness in the group taking timolol was between 1.5 and 3.3 µm, compared with a mean change of between +1.3 and -1.4 µm in the brimonidine group. When assessing longitudinal changes in RNFL thickness, the resolution capability and test – re-test variability of the machine used needs to be taken into account.
Brimonidine is available in New Zealand as Brimonidine 0.2% and Alphagan P 0.15%. The potential for neuroprotection with brimonidine might be an added benefit. It may be prudent to consider brimonidine as an early line therapeutic agent in patients with glaucoma and IOP in the ‘normal’ range. However, it is important to be aware of the high rates of brimonidine allergy.
Betaxolol
Betaxolol is a 1-selective antagonist, and lowers IOP by reducing the production of aqueous humour. The neuroprotective effect of betaxolol is thought to be mediated by inhibition of calcium and sodium ion influx into neurons. In one double-masked study of 40 patients with open angle glaucoma (IOP 24 mmHg), reviewed six-monthly for 18 months, patients were treated twice a day with either betaxolol 0.5% or timolol 0.5%. Although the group treated with timolol had a slightly greater reduction in IOP, the treatment effect on the visual field was greater in the betaxolol group. Another study found no statistically significant difference, at two years, in visual field between patients taking timolol 0.25% and betaxolol 0.5%. There have not been any large clinical trials investigating the neuroprotective effect of betaxolol in glaucoma.
Betaxolol, available in New Zealand as Betoptic eye drops (0.25% or 0.5%), has shown some promise as a potential neuroprotective agent. Presently, betaxolol is used for its IOP-lowering effect, and the possibility of neuroprotection is an additional advantage.
Ginkgo biloba extract
Ginkgo biloba extract has been trialled as a neuroprotective agent in a number of disorders associated with ageing. Ginkgo biloba extract has antioxidant and vasoregulatory effects and has shown promise in slowing retinal ganglion cell death in pre-clinical studies. There has been one prospective, randomised, placebo-controlled, double-masked cross-over study of ginkgo biloba extract, investigating its effect on 27 patients with bilateral normal tension glaucoma and progressive visual field loss. Following four weeks of ginkgo biloba supplementation (40 mg three times per day), visual field mean deviation improved (-11.4 ± 3.27 dB at baseline vs. 8.78 ± 2.57 dB after ginkgo course, p = 0.0001). There was no significant difference between baseline visual fields and those performed post-placebo. Clinically, ginkgo biloba extract is available in various supplement formulations. It is used by some glaucoma specialists in the treatment of glaucoma patients who progress despite low treated IOPs. Larger, longitudinal clinical trials are needed to determine the efficacy of ginkgo biloba extract as a neuroprotective agent in glaucoma.
Memantine
Memantine acts on the glutamatergic system by blocking NMDA receptors that may play a role in retinal ganglion cell death. It is used in the treatment of Alzheimer’s disease (available in NZ as the oral medication, Ebixa). It has shown neuroprotective effects in monkey experimental glaucoma. However, a Phase 3 clinical trial failed to reach its primary endpoint. Although glaucoma progression was slower in high dose memantine compared with low dose, there was no difference compared with placebo.
There are a number of other potential neuroprotective agents in glaucoma, including antioxidants, calcium channel blockers, neurotrophic factors, nitric oxide synthase inhibitors and rho kinase inhibitors. There is currently limited high-quality evidence of neuroprotection for commercially available glaucoma treatment agents.
Ocular perfusion pressure
The vascular theory of glaucoma considers the disease to be the result of insufficient blood supply to the optic nerve. The role of blood pressure in glaucoma has attracted considerable attention, as it represents a clinically modifiable risk factor. Ocular perfusion pressure (OPP) is the pressure available to drive blood through the intraocular vasculature. Mean OPP can be roughly calculated with the following equation, where DBP is diastolic blood pressure and SBP is systolic blood pressure:
Mean OPP = 2/3[DBP + 1/3(SBP-DBP)] - IOP
Some studies specifically measure systolic (SBP – IOP) or diastolic (DBP – IOP) OPP. Diastolic OPP gives information about the lowest OPP values and is thought to be an independent risk factor for open angle glaucoma. Low DPP is the result of low systolic blood pressure and/or high IOP. Several large studies have looked at the relationship between OPP and glaucoma. A summary is given in Table 1.
|
Reference |
Number of participants |
Study design |
Main outcomes |
Adjustment for IOP |
|
Baltimore Eye Survey |
5308 |
Population-based prevalence survey |
6 x increased risk of POAG with DOPP < 30 mmHg compared with > 56 mmHg |
No |
|
Los Angeles Latino Eye Study |
6130 |
Cross-sectional population based study |
Lower MOPP (≤ 50 mmHg), DOPP (≤ 40 mmHg) and SOPP (≤ 80 mmHg) associated with increased OAG prevalence. Very high SOPP (≥ 150 mmHg) also associated with increased OAG prevalence |
Yes |
|
Singapore Malay Eye Study |
3280 |
Cross-sectional population based study |
Lower MOPP and DOPP associated with increased prevalence of OAG |
Yes |
|
Low Pressure Glaucoma Treatment Study |
127 |
Prospective cohort study |
Lower mean OPP associated with glaucomatous visual field progression |
Yes |
Table 1: Main outcomes of studies investigating the relationship between ocular perfusion pressure and glaucoma
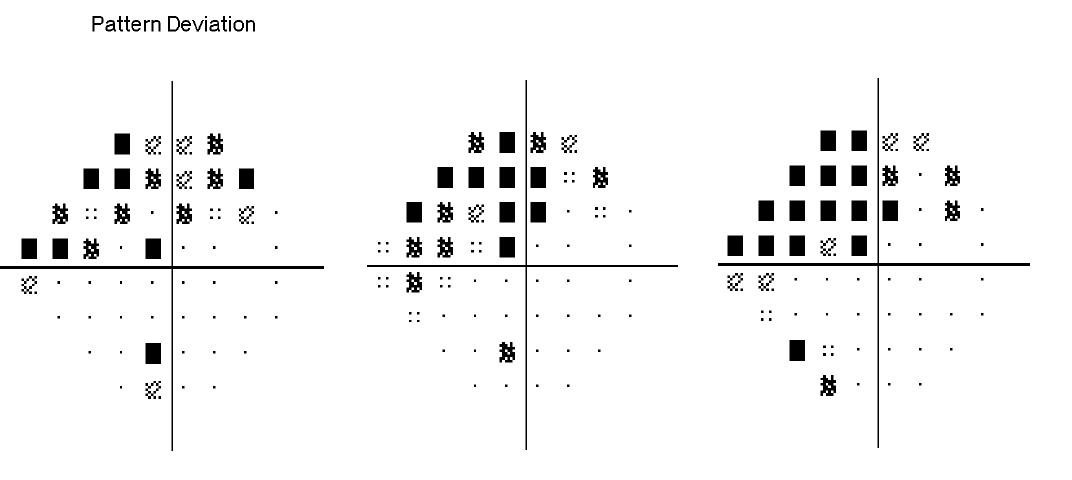
The relationship between OPP and open angle glaucoma is complex. Patients with either low or high OPP have been shown to be at greater risk of glaucomatous visual field progression. This should prompt the clinician to consider patients’ blood pressure, which is not routinely measured as part of a glaucoma assessment. Nocturnal hypotension and fluctuations in OPP have also been shown to be related to glaucomatous disease progression. This may help to explain why some patients continue to have structural and functional deterioration despite clinically adequate IOP lowering (Figure 1.).
Conclusion
Ophthalmologists and optometrists involved in the management of glaucoma should take into account non-IOP factors in glaucoma treatment. This may include the selection of topical anti-hypertensive treatments with potential neuroprotective benefits in suitable patients, possible supplement recommendations as adjunctive therapy, or more rigid monitoring of blood pressure in consultation with the patient’s primary healthcare provider.
About the author
Dr Hannah Kersten is a therapeutically qualified optometrist, currently working as a lecturer in the School of Optometry and Vision Science at the University of Auckland, and in private practice with a focus on glaucoma. Her main area of research interest is ophthalmic imaging in neurodegenerative disorders and glaucoma.
References
Mathan JJ, Patel DV, McGhee CNJ, Patel HY. Analysis of Glaucoma Subtypes and Corresponding Demographics in a New Zealand Population. Biomedicine Hub 2016;1:6-6.
Guymer C, Wood JP, Chidlow G, Casson RJ. Neuroprotection in glaucoma: recent advances and clinical translation. Clinical & Experimental Ophthalmology 2018;13:13.
Vasudevan SK, Gupta V, Crowston JG. Neuroprotection in glaucoma. Indian J Ophthalmol 2011;59:S102-S113.
Yoles E, Wheeler LA, Schwartz M. Alpha2-adrenoreceptor agonists are neuroprotective in a rat model of optic nerve degeneration. Invest Ophthalmol Vis Sci 1999;40:65-73.
Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S, Low-Pressure Glaucoma Study G. A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. American Journal of Ophthalmology 2011;151:671-681.
Tsai JC, Chang HW. Comparison of the effects of brimonidine 0.2% and timolol 0.5% on retinal nerve fiber layer thickness in ocular hypertensive patients: a prospective, unmasked study. J Ocul Pharmacol Ther 2005;21:475-482.
Cantor LB. Brimonidine in the treatment of glaucoma and ocular hypertension. Therapeutics & Clinical Risk Management 2006;2:337-346.
Chidlow G, Melena J, Osborne NN. Betaxolol, a beta(1)-adrenoceptor antagonist, reduces Na(+) influx into cortical synaptosomes by direct interaction with Na(+) channels: comparison with other beta-adrenoceptor antagonists. British Journal of Pharmacology 2000;130:759-766.
Messmer C, Flammer J, Stumpfig D. Influence of betaxolol and timolol on the visual fields of patients with glaucoma. American Journal of Ophthalmology 1991;112:678-681.
Vainio-Jylha E, Vuori ML. The favorable effect of topical betaxolol and timolol on glaucomatous visual fields: a 2-year follow-up study. Graefes Arch Clin Exp Ophthalmol 1999;237:100-104.
Shi C, Liu J, Wu F, Yew DT. Ginkgo biloba extract in Alzheimer’s disease: from action mechanisms to medical practice. International Journal of Molecular Sciences 2010;11:107-123.
Sena DF, Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst Rev 2013:CD006539.
Quaranta L, Bettelli S, Uva MG, Semeraro F, Turano R, Gandolfo E. Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology 2003;110:359-362; discussion 362-354.
Cybulska-Heinrich AK, Mozaffarieh M, Flammer J. Ginkgo biloba: an adjuvant therapy for progressive normal and high tension glaucoma. Molecular Vision 2012;18:390-402.
Gabelt BT, Rasmussen CA, Tektas OY, et al. Structure/function studies and the effects of memantine in monkeys with experimental glaucoma. Invest Ophthalmol Vis Sci 2012;53:2368-2376.
Sena DF, Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst Rev 2017;1:CD006539.
Chung HJ, Hwang HB, Lee NY. The Association between Primary Open-Angle Glaucoma and Blood Pressure: Two Aspects of Hypertension and Hypotension. BioMed Research International 2015;2015:827516.
Zheng Y, Wong TY, Mitchell P, Friedman DS, He M, Aung T. Distribution of ocular perfusion pressure and its relationship with open-angle glaucoma: the singapore malay eye study. Invest Ophthalmol Vis Sci 2010;51:3399-3404.
Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch Ophthalmol 1995;113:216-221.
Memarzadeh F, Ying-Lai M, Chung J, Azen SP, Varma R, Los Angeles Latino Eye Study G. Blood pressure, perfusion pressure, and open-angle glaucoma: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci 2010;51:2872-2877.
De Moraes CG, Liebmann JM, Greenfield DS, et al. Risk factors for visual field progression in the low-pressure glaucoma treatment study. American Journal of Ophthalmology 2012;154:702-711.
Lee J, Choi J, Jeong D, Kim S, Kook MS. Relationship between daytime variability of blood pressure or ocular perfusion pressure and glaucomatous visual field progression. American Journal of Ophthalmology 2015;160:522-537.e521.