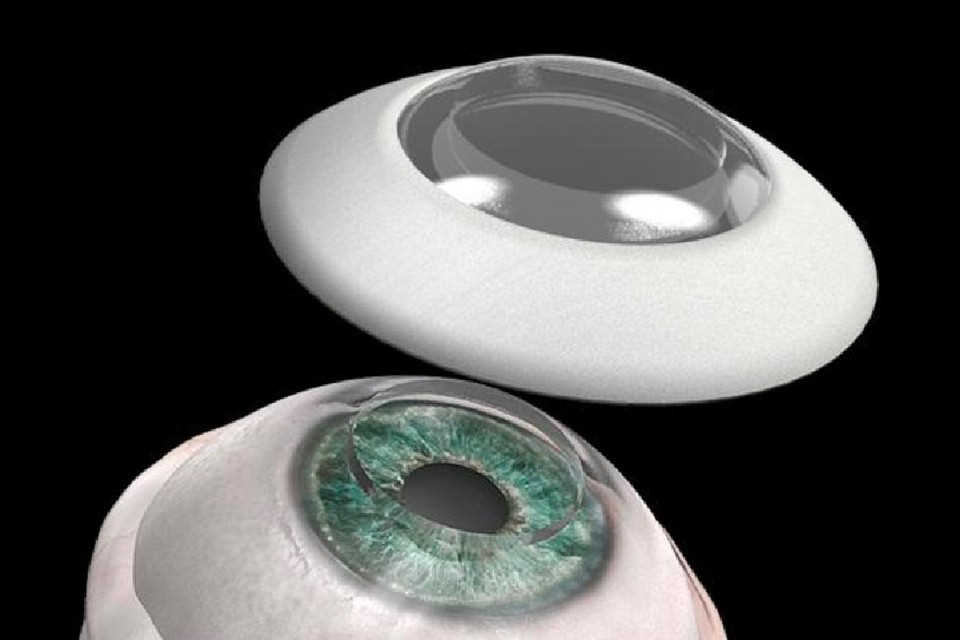
Glazing the windows to the soul
Corneal transplantation was the first, and is still the most frequently performed, human transplant procedure. Yet donor corneas are available for just one in 70 patients worldwide, with 53% of the world’s population unable to access this vital tissue, according to a 2016 paper by Professor Philippe Gain et al from the University Hospital of St-Etienne, France.
Globally, the top indications for corneal transplants are Fuchs’ dystrophy (39%) keratoconus (27%) and sequellae of infectious keratitis (20%), reported Prof Gain. In New Zealand, where donor cornea shortfall is around 50%, keratoconus accounted for more than 40% of cases, the highest reported proportion of transplantation surgery for keratoconus worldwide. However, with the advent of corneal crosslinking (CXL), this has fallen significantly, said Professor Charles McGhee, chair of the New Zealand National Eye Bank and of ophthalmology at the University of Auckland, in a 2021 study.
As well as the cultural problems surrounding cornea donation (see 'Why the dearth of donated corneas?' below), more than 50% of the human population lives in countries without tissue banks or corneal specialists, said Dr Gilad Litvin, ophthalmic surgeon and founder, chair and chief medical officer of Israeli synthetic-cornea company CorNeat Vision. “There are also about 20-25% of patients who are not suitable for transplant even if donor tissue was available,” he said.

Dr Gilad Litvin
It’s this combination of donor cornea shortfall and graft-rejection risk that has researchers around the world pursuing alternatives, from 3D-printed or lab-grown corneas to fully synthetic prosthetics (keratoprosthesis or KPro) and cell reprogramming techniques. Those who succeed will save vision, improve lives and secure themselves a significant share of the global artificial cornea and corneal implant market, which is projected to reach US$656.19 million by 2028, according to a 2021 report by Allied Market Research. As Dr Litvin put it, “There’s a lot riding on the success of artificial corneas.”
The synthetic solution
In January 2021, CorNeat announced it had implanted the first human, 78-year-old Jamal Furani, with a fully synthetic cornea. Furani’s oedema had left him blind for a decade. Earlier this year, Dr Litvin visited Furani at home, and said he saw how independent he’d become. “He has retained the same vision (as when he’d first received the transplant) – which is a bit compromised due to the fact that he’s diabetic and he has retinal issues – but the optics and integration concept have been proven, now he’s over a year post-implantation. Prior to this, he hadn’t seen for a decade, so it’s very dramatic.”
The implant is fully synthetic, with no stem cell involvement, he said. “There have been no issues with rejection because, as a completely inert polymer substrate, it doesn’t create an inflammatory response. However, there are issues with integration – it took a few attempts to perfect the configuration of the device to fit the anatomy, and making the procedure feasible for the surgeon to implant it was extremely difficult.”
Seven further patients have since received the CorNeat KPro and additional implantations are planned in the coming months. CorNeat also intends to initiate a multicentre clinical trial to gain the European CE mark and approval from the US Food and Drug Administration, said Dr Litvin. “We hope to have approval towards the end of 2023. We also started discussions with China, where there’s a very big shortage of tissue and a lot of demand; they have the facilities but they don’t have a lot of donations. There are over five million patients in line but barely 5,000 transplantations a year.”
Five months after Furani’s ground-breaking transplant, and also in Israel, EyeYon Medical’s EndoArt synthetic cornea received CE approval. EyeYon co-founder and CEO Nahum Ferera said implantation of his company’s corneal polymer film is a minimally invasive surgical procedure and might help avoid or delay endothelial transplantation in patients with endothelial dysfunction. Although it may help reduce the demand for donor corneas, EndoArt does not replace the patient’s cornea and is currently only used to treat corneal oedema.
Over in South Korea, in August 2021, the country’s Ministry of Food and Drug Safety granted innovative medical device designation to Te Bios Co’s C-Clear, bringing it closer to becoming the first artificial cornea to be approved there. C-Clear consists of a core that transmits light to secure a field of view and a skirt with a porous structure that helps connect with tissue while minimising inflammatory response, according to a study in the Korea Biomedical Review.
Saying no to plastic
Taking a different tack, Dr Farshid Sefat, associate professor at the Faculty of Engineering & Informatics at the University of Bradford, UK, said his team is close to perfecting a biopolymer cornea ‘scaffold’ on which to grow regenerative stem cells. The new system would create multiple layers, more closely mimicking the eye’s natural design, and is around 500μm thick. This technology may cure blindness caused by damage to the cornea, he said.

Associate Professor Farshid Sefat
Closer to home, BIENCO, an international consortium launched in December 2021 and led by the University of Sydney’s Professor Gerard Sutton, is focusing on developing bioengineered eye tissue to treat corneal blindness. “BIENCO is looking at a totally bio-engineered cornea with no plastic bits. But also in the pipeline is our plan to produce layers of the cornea that can address specific corneal diseases,” said Prof Sutton, adding that in his experience all prosthetic KPros share the problem of tissue integration. “(In New Zealand and Australia) keratoconus is being treated successfully with CXL but is still an issue around the world. Fuchs’ dystrophy is treated with a single-layer cell replacement, which we are bio-synthesising. In the developing world, however, infection and trauma are the most common indications and require a full-thickness graft. We are currently sourcing human collagen from skin and transforming it into clear collagen for corneas. We can bioprint cells within that structure. We hope to be successful in our second round of funding this year, but a product on the shelf is five years away.”

Professor Gerard Sutton
Turning back time
Exploring several non-plastic routes is Professor Trevor Sherwin and his team at the University of Auckland. “In my view, the idea of growing an artificial cornea in a dish then transplanting it into a patient is a slightly archaic and complex solution. A lot of that thinking came about when penetrating keratoplasty was the go-to operation but we didn’t have enough corneas.”

Professor Trevor Sherwin
Corneal surgeons are doing a lot more component surgery now, such as Descemet’s stripping endothelial keratoplasty (DSAEK) and Descemet’s membrane endothelial keratoplasty (DMEK), said Prof Sherwin. So he’s more interested in providing a toolbox of potential therapeutics that the eye specialist can choose from, “Rather than growing a whole cornea just to repair, say, a sick endothelium,” he said.
The Auckland University team is using adult stem cells isolated from corneal tissue. Like A/Prof Sefat in the UK, they are looking at building novel scaffolds, said Prof Sherwin, which can be used as carriers to deliver cells to the cornea or on which cells can grow. “The scaffolds are made from crystallins obtained from hoki fish lenses. We reasoned that if we could use the crystal lens to make a new scaffold, then we should have a corneal tissue which would have optically good properties and very little immunogenic response.”
Perhaps the most elegant of Prof Sherwin’s potential solutions is cell reprogramming. “When axolotls lose a limb, people used to think the mechanism for repair was a flow of stem cells from the body to the injury site and they’d start regrowing the limb. But the cells at the injury site actually reprogramme to an earlier development time, so they can start making bone and blood vessels and nerves from scratch. Somewhere along the line, humans lost the ability to do that. But we haven’t lost the molecules to do that and we’ve shown they still have the ability, given the right stimulant. Essentially, a cornea is a fairly easy tissue to remake, if you can reprogramme the cells correctly to say ‘make a cornea again’.”

An axolotl
By treating adult corneal cells with a growth factor and steroid cocktail, the team managed to make them express a collagen they usually only make in the foetal cornea. They applied this to keratoconic tissue and it too made this new collagen and began thickening the cornea, said Prof Sherwin. “We did that experiment in vitro and in sheep and showed that by supplying this growth factor and the steroid, the cells start making this collagen type, but as soon as we withdraw it, the de novo collagen synthesis decreases. We believe the cells then revert to type. The beauty of this sort of reprogramming is that you shouldn’t get the same possibilities of introducing rogue, cancer-forming cells that we worry about with stem-cell implants.”
Compared to synthetic corneas that are bespoke for each patient, cell reprogramming could be used by many patients, said Prof Sherwin, explaining that with corneal transplantation, if the patient rejects it, you can use ABO blood-group typing, which could be easily covered through a handful of cell lines. “If you go to human leukocyte antigen (HLA) matching, you might need a few more than that, but essentially, you could build a bank of stem cells so you could say, for example, ‘This patient needs cell type 53’.”
A chip on the stroma
After working on corneal tissue engineering for 15 years, in May 2018, Professor Che Connon’s team at the University of Newcastle, UK, announced they had produced corneal stroma using an inexpensive, off-the-shelf 3D-printer. However, it had limitations, said Prof Connon. “That was very much a proof-of-concept work, showing that you could extrude bio-ink containing corneal stromal cells and create an arrangement of cells and bio-ink that had the same shape as the corneal stroma. What it didn’t have was appropriate transparency, mechanical strength or structure, without which we cannot get the functionality.” This, compounded by corneal collagen fibres having a resolution in the tens of nanometres, while 3D printing has a resolution of 50 to 100μm – a thousand times bigger – told Prof Connon’s team that 3D printing looked unlikely to be a solution by itself.
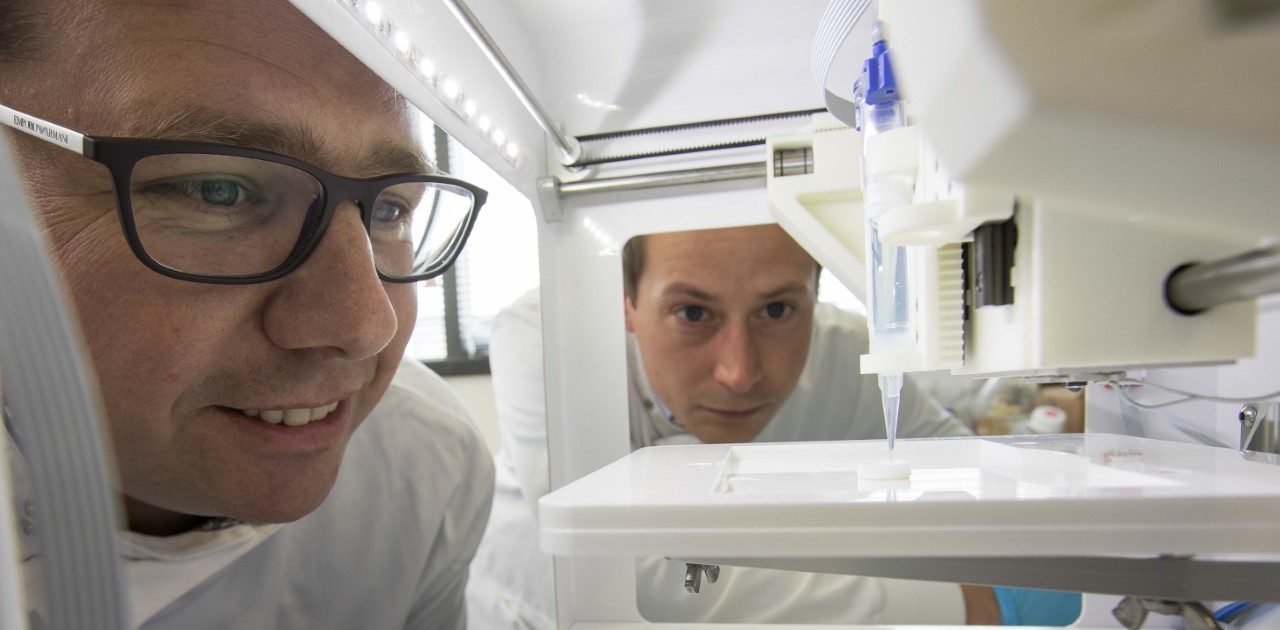
Professor Che Connon (left) and Dr Steve Swioklo 3D-print a 'cornea'
The team then turned its focus towards working out how cells arrange themselves into highly sophisticated tissues with aligned cells producing aligned collagen. “(Being able to replicate) that means you can generate very sophisticated tissues that are identical to the native corneal stroma,” said Prof Connon. This technology has now been spun out into its own company, 3D Bio Tissues, which is in the process of developing a clinical trial to bring these lab-grown corneas to market.
“We’re also making really amazing progress with the engineered corneal stroma using what we call our tissue-templating platform,” said Prof Connon. “Because we build the cornea up, we can embed things as it’s built. We have a PhD student working on how to power a chip that can be embedded into a lab-grown cornea. That’s a big step, because you could use a chip for, say, measuring intraocular pressure, or you could put a camera in there and the camera could be pointing back into the eye and looking at certain things there, or looking outwards.” One of the hurdles with bioelectronics is the integration of the chip into the host tissue, which often causes rejection, but if you could embed it into an engineered cornea, you could solve some of those integration issues, he said, so you shouldn’t have any problems beyond the normal corneal transplant issues. “We aim to produce lab-grown corneas that are identical to donor corneas, in terms of the cell type, the collagen arrangement and the other extracellular matrix components, and its mechanical properties, such that a surgeon would not be able to tell the difference between the tissues we are generating and the donor tissues they’re used to dealing with.”
Why the dearth of donated corneas?
The act itself of donating is one of the barriers to cornea supply, wrote Dr Aaron Ong, Professor Charles McGhee, Nigel Brookes and Dr Jie Zhang in May 2022’s NZ Optics. “Unlike our counterparts in Australia, who actively screen all deaths as potential eye donors via their DonateLife network, there is a lack of similar donor screening programmes here (in New Zealand).”*
In New Zealand in 2020 there were 119 eye donations, equating to roughly 24 donations per million population, compared to 419 per million in the US and 87 per million in the UK in 2016. Unfortunately, the number of donated corneas alone is not the full picture – a 2020 paper by Professor Jodhbir Mehta et al, Singapore National Eye Centre, said about a third of donor corneal tissues harvested were reported to be not suitable for transplant surgeries due to an abnormal donor infectious screen or low endothelial cell density (ECD), which falls by around 0.6% annually from birth.
In England, an opt-out donor system (where everyone automatically consents to organ donation, unless they formally specify otherwise) was introduced in 2020, but it does not cover limbal stem cells, which are key to corneal regeneration, and relatives of the deceased can still forbid harvesting of donor tissue. In New Zealand, The Human Tissue Act (2008) also requires that informed family consent is obtained before organs or tissues can be removed for donation. “It is rare for a family to ‘overrule’ the wishes of their family member when those wishes are known. It is when there has never been a conversation about donation that some families can struggle to make a decision. This is why we encourage everyone to have a conversation about donation with their family, whānau or close friends now,” said Rebecca Oliver from Organ Donation New Zealand. “New Zealand is not currently considering an opt-out system of organ donation as it has not shown to increase organ and tissue donation rates,” she added.
However, a 2019 University of Michigan simulation found an opt-out system would have added between 4,300 and 11,400 life years for the more than a half a million patients on the US organ transplant list from 2004 to 2014. Further, after switching to an opt-out system in 2015, consent rates for donation reached an all-time high of 77% in 2018-2019 in Wales, having being as low as 58% when the system was implemented. Further validation comes from a 2016 paper by Dr Philippe Gain, from Jean Monnet University and University Hospital in France. Of the 77 cornea-procuring countries his team surveyed, 35 (45%) used an opt-in system and 42 (55%) used an opt-out system, with the corneal procurement rate per capita in the opt-out countries being more than seven times that of those with an opt-in system.
“In some countries, there’s a very big cultural gap in thinking that in the afterlife, they don't want to be blind,” explained CorNeat Vision’s Dr Gilad Litvin. “Where this is the culture, nobody donates. So you need cooperation and education. Perhaps if when famous people die, they donate their organs, there will be a trickle-down effect.”