Corneal transplant rejection: principles and clinical management
Corneal transplantation is the most common form of tissue transplant in New Zealand with more than 300 cases performed annually. As widely-known, the primary indication for corneal transplantation in New Zealand over the last 25 years is keratoconus¹. Fortunately, keratoconus as an indication is typically associated with good long-term survival, being in the region of 90% at 10 years in the Australian Graft Registry. Nevertheless, the most common cause of transplant failure in both penetrating and endothelial transplant is allograft rejection². This brief review will consider the “relative” immune privilege of the cornea, the associations of allograft rejection and the current clinical diagnosis and management options.
Relative immune privilege of the cornea
Corneal transplantation is generally regarded as a highly successful procedure with low rates of rejection due to the immune-privileged status of the cornea. This diminished immunity is due to an accumulation of factors including the absence of blood vessels and lymphatics where immune cells primarily reside, modified corneal antigen expression, the absence of antigen presenting passenger cells in the donor tissue, immune-suppressive molecule secretion, the inhibition of immune effectors by surface cells, and anterior chamber-associated immune deviation. The immune-privileged nature of the cornea explains the unusually high survival rate of transplantation in the quiet eye with topical immunosuppression alone, in contrast to other solid organ allografts which require systemic immunosuppression.
However, in the event of corneal or anterior segment inflammation, this immune privilege can become compromised, pre-disposing the eye to allograft rejection. Inflammation leads to an increase in the leakiness of blood vessels with egress of cells and proteins into the anterior chamber, formation of new corneal blood vessels and lymphatics, increased expression of HLA antigens in the cornea as well an accumulation of bone marrow-derived cells. Therefore, any ocular inflammation must be treated promptly and effectively to maintain corneal privilege.
Rejection and high-risk cases
Although there is no consensus on the exact threshold of what constitutes a ‘high-risk case’, a survey of corneal surgeons in the UK identified the top 10 perceived risk factors: significant corneal vessels, previous episodes of graft rejection in the same eye, previous herpetic eye disease, previous chemical injury, active external eye disease, previous ocular inflammation or infection, dry eyes, glaucoma, previous episodes of graft rejection in fellow eye, and children³.
A multivariate analysis of the Australian Corneal Graft Register⁴ showed the following clinical factors to best predict corneal graft failure: 1) indication for graft; 2) number of previous ipsilateral grafts; 3) eye inflamed at time of graft; 4) larger graft diameter; 5) lens status immediately after graft; 6) neo-vascularisation of the cornea; 7) occurrence of graft rejection episode; 8) microbial keratitis or stitch abscess in the graft; 9) early removal of graft sutures; 10) post-operative rise in intraocular pressure.
Identification of high-risk cases is crucial for risk-to-benefit discussion and to allow for careful planning of perioperative management and post-operative surveillance to maximise the chances of graft survival.
In ‘high-risk transplants’ where the graft is more likely to fail, several management strategies may be employed to minimise the effect of the corneal allograft response. Anti-inflammatory measures such as high-dose topical corticosteroids, HLA matching of graft to host and systemic immunosuppression agents such as corticosteroids/cyclosporine/tacrolimus/mycophenolate and azathioprine may be considered. Other strategies may also include maximising corneal lubrication, stabilising the intraocular pressure, treatment of new vessels, if possible, and planning for suture management.
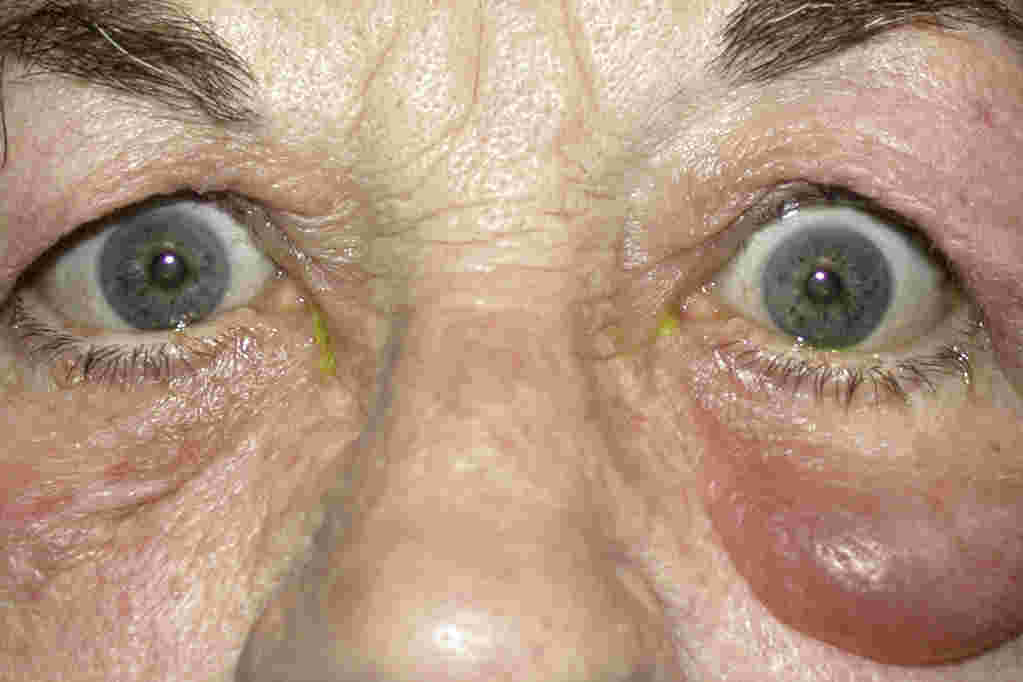
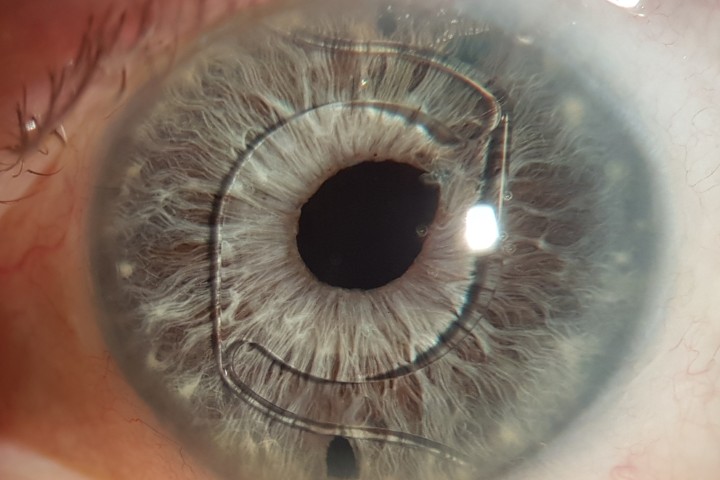
Fig 2. Early endothelial rejection with multiple fine infero-temporal cellular deposits, coalescing into an early diagonal immune line, with very early stromal but no epithelial oedema
Identifying allograft rejection
It is important to always have a high index of suspicion for rejection and monitor high-risk cases closely. Patients’ symptoms, such as decreased vision, photophobia and redness, are important clues to aid diagnosis. On examination there may be a reduction of visual acuity, increased redness, corneal oedema, cells and flare in the anterior chamber and keratic precipitates.
Corneal graft rejection can be broadly divided into three types: epithelial, stromal and endothelial. Epithelial rejection occurs early in the post-operative course, usually within three months. It manifests with poor quality, grey oedematous epithelium. Due to their mild, transient nature, epithelial rejection episodes are often missed and do not typically lead to graft failure because of limbal migration of new host epithelium. The eye appears quiet or pink, with no anterior chamber activity, and treatment with topical prednisolone acetate 1%, four times daily is usually sufficient.
Stromal rejection causes a mild to moderate reaction and typically presents with sub-epithelial infiltrates or Krachmer’s spots (which can mimic the appearances of adenoviral infection limited to the donor cornea only). Although this does not typically lead to graft failure, it represents sensitisation of the host to the graft and should be aggressively treated to prevent progression to endothelial rejection.
Endothelial rejection is by far the most common and serious form and can lead to graft failure in days without prompt treatment. It typically manifests with anterior chamber cells, fine endothelial cellular deposits or keratic precipitates, occasional endothelial immune lines (Khodadoust’s line), corneal oedema and variable circumcorneal injection.
Treatment of allograft rejection
Acute allograft rejection warrants urgent specialist referral to maximise chances of graft survival. Treatment usually involves intensive, hourly topical prednisolone acetate 1% and dexamethasone ointment at night. In cases of severe endothelial rejection, systemic steroid therapy with intravenous methyl-prednisone (3-5mg/kg) or oral prednisone (1-1.5mg/kg) may also be considered. Following the acute episode, topical corticosteroids are often continued longer term to reduce the likelihood of further rejection. However, treatment with corticosteroids is not without risk and patients must be fully informed and closely monitored. Well-known risks of corticosteroids include ocular side effects of raised intraocular pressure (IOP), cataract formation, predisposition to microbial keratitis, and systemic side effects of hyperglycaemia, hypertension, weight gain, osteoporosis and gastritis.
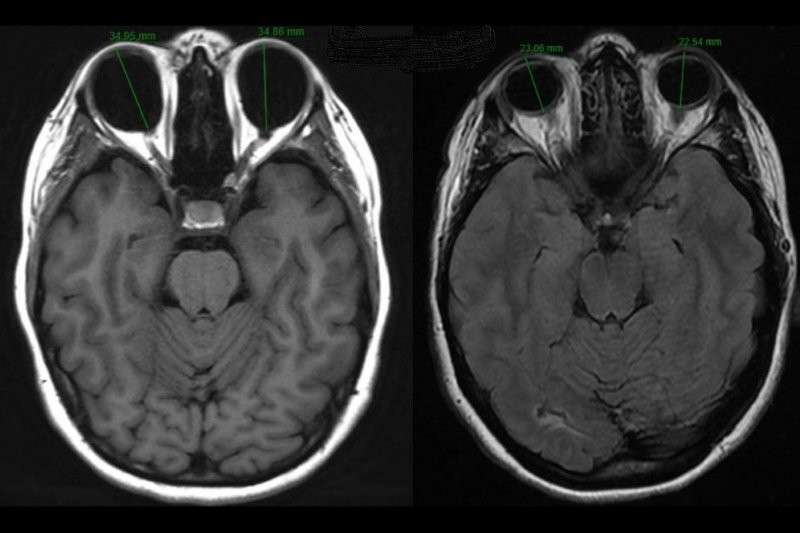
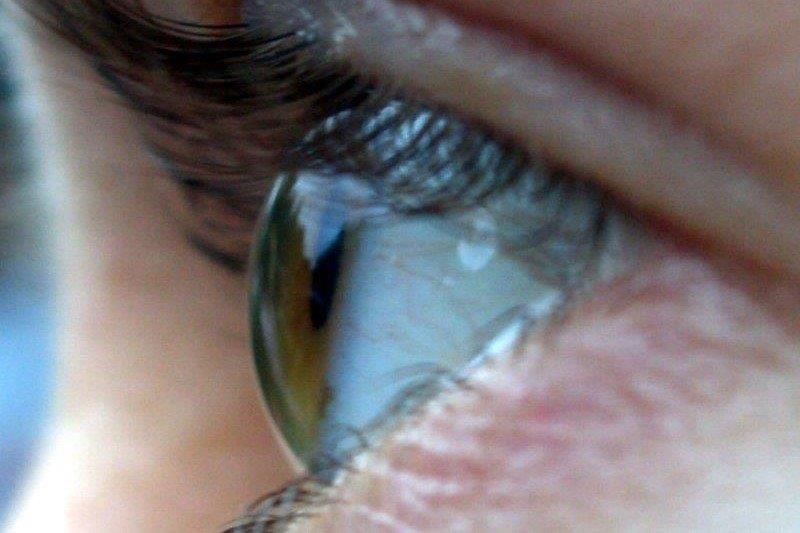
Fig 3. Established, severe endothelial rejection with multiple keratic precipitates, Descemet’s folds, stromal and epithelial oedema
Conclusion
To maximise the success of corneal transplantation, a practitioner must be vigilant for any risk factors of rejection which may be ameliorated pre- or post-operatively. It is important to recognise the symptoms and signs of graft failure early and provide urgent treatment to maximise chances of halting and reversing a rejection episode. Topical corticosteroids, sometimes in combination with systemic corticosteroids, are still the primary treatment choice for acute corneal graft rejection. In high-risk cases, single and combined administration of systemic immune suppressive agents, such as tacrolimus, cyclosporine and mycophenolate, appear to be promising in prolonging graft survival. However, patients must be well informed regarding the risks of treatment and these must be weighed against the benefits. In the future, cellular and molecular therapies should allow us to achieve immunologic tolerance even in high-risk grafts.
References
- Kim BZ, Meyer JJ, Brookes NH, Moffatt SL, Twohill HC, Pendergrast DG, Shwerwin T, McGhee CNJ. New Zealand trends in corneal transplantation over the 25 years 1991-2015. Br J Ophthalmol. 2017;101(6):834-838.
- Crawford AZ, Krishnan T, Ormonde SE, Patel DV, McGhee CN. Corneal Transplantation in New Zealand 2000 to 2009. Cornea. 2018;37(3):290-295.
- Koay PY, Lee WH, Figueiredo FC. Opinions on risk factors and management of corneal graft rejection in the United Kingdom. Cornea. 2005;24(3):292-6.
- Coster DJ, Williams KA. Management of high-risk corneal grafts. Eye (Lond). 2003;17(8):996-1002.
About the authors
Dr Jina Han is a cornea and anterior segment clinical research fellow at Auckland University. She completed her medical degree at Otago University and is currently pursuing her MD on risk stratification and outcomes of cataract surgery.
Professor Charles McGhee is chair of ophthalmology at Auckland University, current president of the Asia Pacific Academy of Ophthalmology and founding director of the NZ National Eye Centre. He specialises in corneal diseases, including dystrophies and keratoconus, has performed more than 1000 corneal transplants and published more than 300 peer-reviewed articles.