Eylea funded
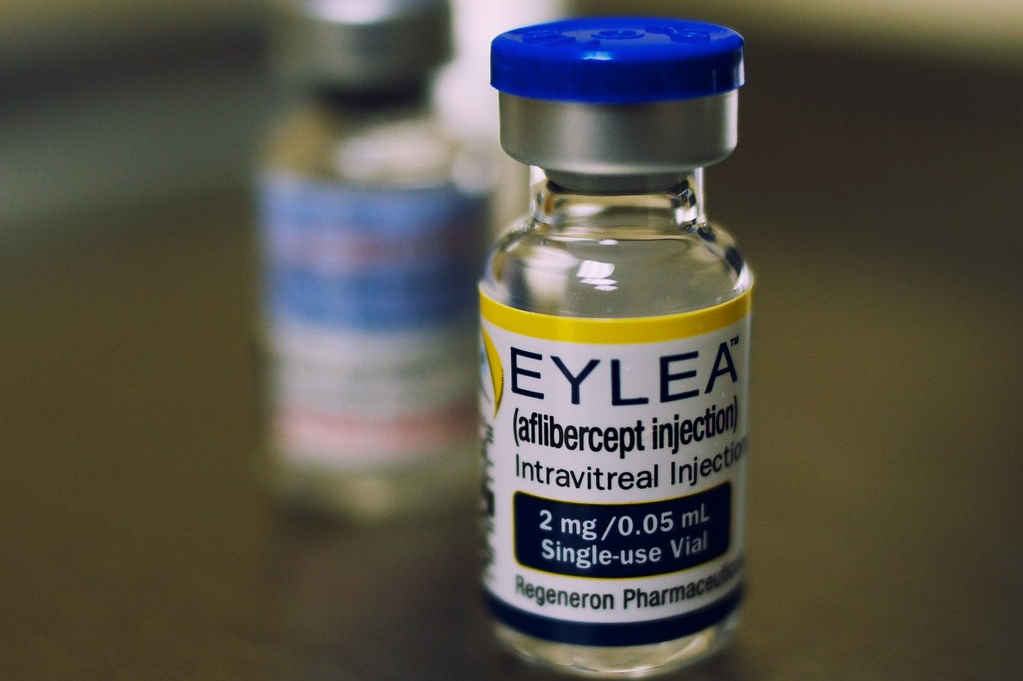
After several meetings, rejections and new requests for feedback, aflibercept (Eylea), used to treat degenerative eye conditions, has finally been given the funding go-ahead from 1 June this year, for both hospitals and the community.
“This is a new generation treatment that helps preserve and improve vision,” said Lisa Williams, Pharmac director of operations, in a media release announcing the decision. “It increases treatment options for people with wet age-related macular degeneration (wAMD) and diabetic macular oedema (DMO), both of which are extremely common causes of blindness in New Zealand.”
wAMD is New Zealand’s most common cause of blindness, affecting one in seven people over the age of 50. DMO is a serious complication of type 1 and 2 diabetes and is the leading cause of blindness in people aged 25–74.
“Aflibercept will provide more flexibility for patients and reduce the burden on DHB ophthalmology services as it requires less frequent injections than the current treatments, and will be funded whether prescribed by private or public ophthalmologists,” said Williams. “We expect this to have a positive impact on the wider health system, while providing a better treatment option for patients.”
Around 900 people are expected to benefit from this decision in the first year of funding.
For the full media release and related documents, visit: www.pharmac.govt.nz/news/