Imaging in The Big Apple
Early this year, I was awarded a Claude McCarthy Fellowship to undertake original research abroad and attend an international conference. I was delighted to be granted this fellowship and to have the opportunity to travel to New York to spend time at Stony Brook University as a result.
Being a physics graduate, my expertise is in magnetic resonance imaging (MRI), a very powerful and non-harmful imaging modality that’s widely used for clinical diagnosis and biomedical research. The application of MRI for the human eye is a relatively new area, but one in which there has been steady progress over the past few years.
Understanding presbyopia and cataract with MRI
My PhD uses MRI to assess the physiological optics of the crystalline lens and to understand how lens ageing progresses into presbyopia and cataracts. These may sound like two entirely separate topics, but the linkage between MRI and the lens is hydrogen. Hydrogen is the most abundant element in the human body and MRI uses it to generate an image signal, and the eye’s crystalline lens is made up of water and protein, both containing tons of hydrogen. Thus, if we employ reverse engineering to look at the signal intensity in the MRI images, we can gauge the amount of water and protein content that exists in the lens.
The key hypothesis of our research is that the age-related changes of the underlying cellular functions and communication between cells results in lens cells being unable to effectively exchange nutrients.
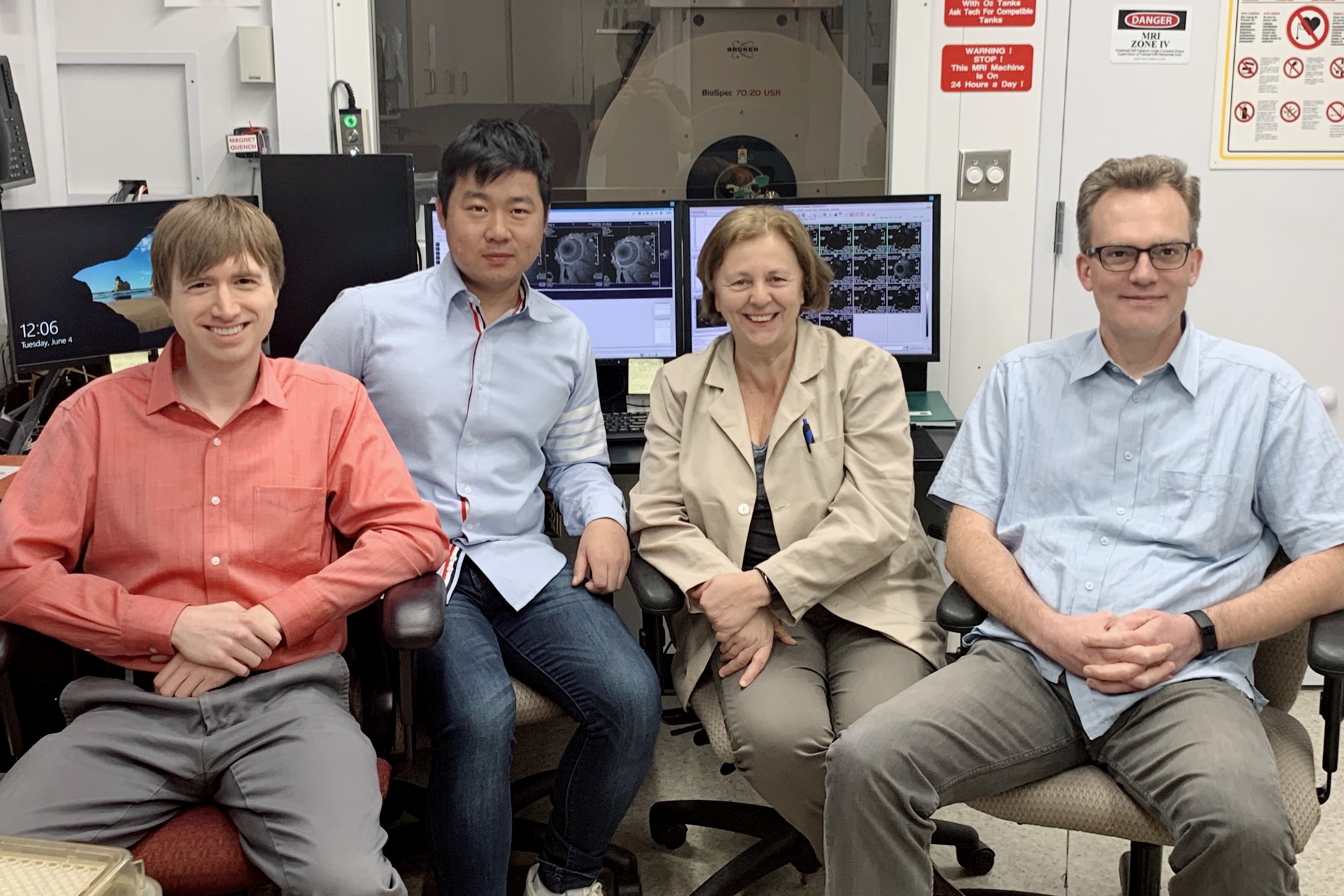
The Molecular Vision Lab at the University of Auckland, led by Professor Paul Donaldson, is an internationally renowned group that researches the lens, employing a number of talented biologists, physicists, biochemists and engineers. In 2016, our lab, together with the lab led by Professor Thomas White who heads up Stony Brook’s department of physiology and biophysics, was awarded a US National Institute of Health (NIH) grant to establish a collaborative research project to investigate age-related changes in the lens from a cellular level to tissue level. This includes my work in Auckland and the US team’s genetically modified mice model, which employs Stony Brook’s state-of-the-art, 7T pre-clinical (animal) MRI to manipulate different cellular functions within the lens.
I came to Stony Brook last year to establish the same protocols we use for human studies on their animal MRI and again this year to apply these technologies to image genetically modified mice.
In Prof White’s own words, “We developed MRI protocols to study lens water content and water/protein ratio non-invasively in human subjects. Our observations show that these parameters change in an age-dependent manner and can be used as physiological biomarkers to monitor the onset of age-related nuclear cataract. Extending these studies to genetically engineered mouse models of cataract will help gain mechanistic insight into how lens transport fails with age.”
Initial results look very interesting and exciting and I believe they will help us better understand the mechanism of cataract and provide new insights for cataract research. I hope to publish my results later this year.
The experience: Stony Brook and NY
At first, I was just like a ‘fanboy’ when I saw the MRI machine here and was given the opportunity to work with people whose names I’d only previously seen published!
Stony Brook itself is a small town in Long Island, about one and half hours by train from New York City. Like the small towns near Auckland, life here is quiet and the people are very friendly.
For a Kiwi researcher, this was not only a great opportunity to undertake advanced research, but to expand my horizons. I’m very grateful for this opportunity, and the ‘nightmare’ long distance flight is well worth it!
Wilson Pan is a third year PhD student at the School of Optometry and Vision Science at Auckland University. As well as continuing his work at Stony Brook, the Claude McCarthy Fellowship allowed him to attend this year’s ARVO conference where he presented a poster on his and his colleagues’ work into Measuring the fluid viscosity of vitreous body using MRI.