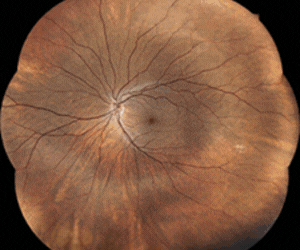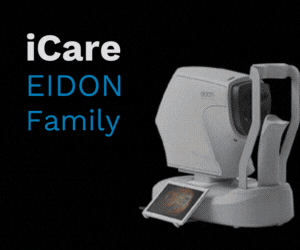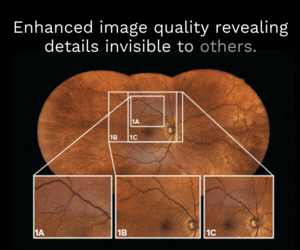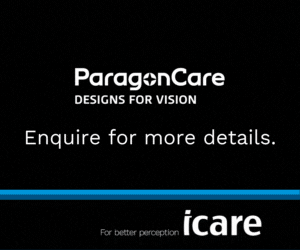
Inflammasome markers in dry eye disease
Dry eye disease (DED) is one of the most common yet undertreated ocular surface conditions. The Tear Film and Ocular Surface Society’s second Dry Eye Workshop (TFOS DEWS II) recognised DED as a multifactorial, self-perpetuating vicious circle characterised by tear film instability and hyperosmolarity, which ultimately leads to chronic ocular surface inflammation and cellular damage1. This results in ocular discomfort and visual disturbance that negatively impact the quality of life of patients2.
Given the multifactorial nature of DED, therapeutic approaches that target a single cause often have suboptimal treatment outcomes in breaking the vicious circle of DED. Inflammation plays a significant role in the progression of the disease3. However, while currently marketed anti-inflammatory eye drops are recognised to relieve DED symptoms in the short term, extended corticosteroid use can cause significant ocular side effects, including cataract and glaucoma; non-steroidal anti-inflammatory drugs can lead to reduced corneal sensitivity4; while formulation additives, including preservatives and surfactants, can cause ocular toxicity, further worsening symptoms.
Reportedly, inflammasomes play a critical role in a number of autoimmune and inflammatory diseases. A recent study by Niu et al5 noted a significant upregulation of the nucleotide binding domain and leucine-rich repeat containing protein-3 (NLRP3) in the tears and ocular surface of DED patients. Although inflammasome inhibitors have been widely investigated, none have been tested for the treatment of DED. As part of a doctoral-level research project, we are investigating the role of the inflammasome and its associated markers Interleukin-1 beta (IL-1b) and IL-18 in tears and conjunctival cells in patients with DED and healthy individuals. Findings will be used to inform the parallel development of a novel ocular treatment.
This study is part of a Health Research Council project grant for investigating a novel eye drop that tackles the vicious circle of DED, awarded to Associate Professor Ilva Rupenthal, director of the Buchanan Ocular Therapeutics Unit, in collaboration with Professor Jennifer Craig and Dr Priyanka Agarwal, from the University of Auckland. Development of such a novel, preservative-free eye drop offers the possibility of eliminating the need for current anti-inflammatory therapies that have significant sequelae, with a view to ultimately improving the quality of life for the up to 1 in 3 people in New Zealand6 and elsewhere affected by dry eye.
References
1. Craig JP, Nelson JD, et al. TFOS DEWS II Executive Summary. Ocular Surface. 2017;15(4):802-812.
2. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocular Surface. 2017;15(3):334-365.
3. Ganesalingam K, Ismail S, Sherwin T, Craig JP. Molecular evidence for the role of inflammation in dry eye disease. Clinical and Experimental Optometry. 2019;102(5):446-454.
4. Singer DD, Kennedy J, Wittpenn JR. Topical NSAIDs effect on corneal sensitivity. Cornea. 2015;34(5):541-543.
5. Niu L, Zhang S, Wu J, Chen L, Wang Y. Upregulation of NLRP3 inflammasome in the tears and ocular surface of dry eye patients. PLoS ONE. 2015;10(5):e0126277
6. Wang MTM, Muntz A, Lim J, et al. Ageing and the natural history of dry eye disease: A prospective registry-based cross-sectional study. Ocular Surface. 2020;18(4):736-741.


Dr Stuti Misra (left) is a lecturer and researcher, focusing on ocular surface abnormalities and corneal imaging, at Auckland University’s Department of Ophthalmology, where optometrist Dian Zhuang (right) is working towards a PhD in ophthalmology.