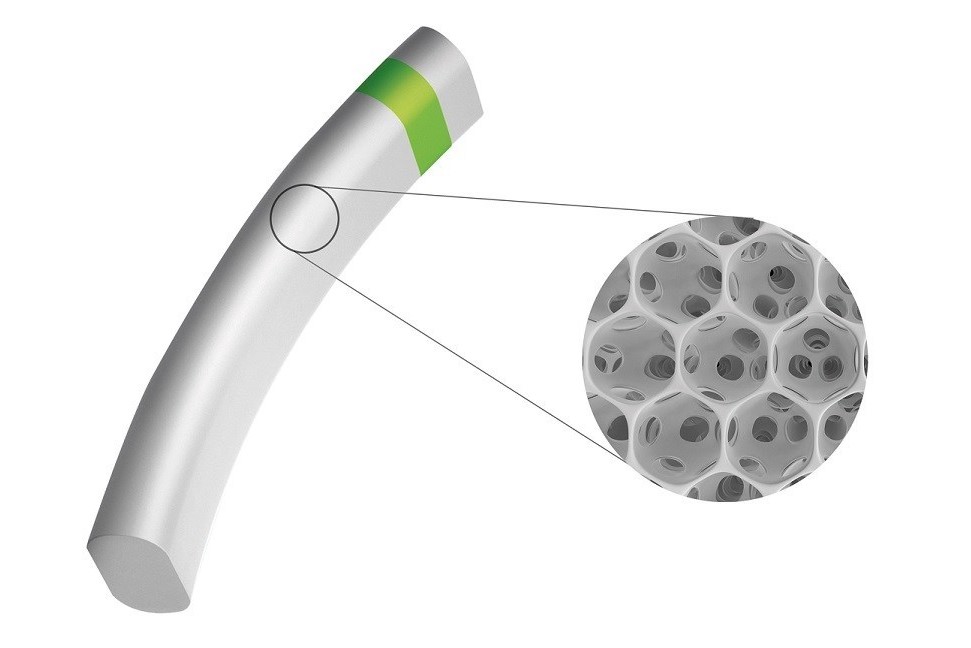
Miniject glaucoma device reports positive trial success
Miniject, iStar Medical's micro-invasive glaucoma surgery (MIGS) device, has shown positive one-year results in its ‘Star II’ European trial.
One year after surgery, 29 patients with open-angle glaucoma had a mean intraocular pressure (IOP) of 15.1mmHg, corresponding to a 38% reduction from medicated baseline IOP, with 45% of them no longer requiring IOP-reducing medication at that point.
The implant, made of the porous ‘Star’ material developed by the University of Washington, USA, has demonstrated excellent bio-integration, allowing aqueous humor to flow freely through it. Studies conducted in rabbits – an animal model known for its aggressive inflammatory response – demonstrated that Miniject bio-integrated with surrounding tissue with no observed fibrosis, implant encapsulation, nor dense connective tissue obstructing drainage channels. Researchers say that this may result in the implant affording enduring IOP reduction.
"The Star-II results at one year confirm Miniject is a remarkably effective treatment option for patients with glaucoma. It is encouraging to see that the positive six-month results have been sustained at one year," said Professor Julián García-Feijoó, one of the study’s investigators from Complutense University, Madrid.