Ocular manifestations in Down Syndrome
Down Syndrome (DS), trisomy of chromosome 21, is one of the most common genetic causes of intellectual disability. It affects 50-80 newborns annually in New Zealand, with advanced maternal age being the primary risk factor. This article encompasses the ophthalmic impairment and clinical considerations for individuals with DS, however, other co-morbidities include hearing impairment, cardiac and respiratory anomalies.
Emmetropisation
The mean refractive error at birth among DS and non-DS populations are reported to be similar. Emmetropisation, the mechanism by which refractive power and axial length is naturally adjusted to approach emmetropia during childhood development, may be impaired in DS. Woodhouse et al. showed that 50% of pre-school and 54% of primary school children with DS have spherical equivalent outside -0.75DS to +3.00DS. In contrast, 5.8% of pre-school and 3.2% of primary school children in the general population have spherical equivalent outside the specified range. Impaired emmetropisation has been attributed to reduced accommodation, reduced near work, and abnormalities in the cortex.
Refractive error
Hyperopia, astigmatism and anisometropia are more prevalent in children with DS. Significant astigmatism (1.00DC) tends to be more prevalent in DS children with an increasing magnitude in early years and young adult life. Given impaired emmetropisation and higher prevalence of refractive error among DS, approximately 60% of affected children require spectacles. Among adults with DS, myopia and astigmatism are the main types of refractive error. Increased myopia prevalence among adults may be due to cataract and keratoconus. Facial characteristics in DS include a flat nasal bridge and small and low set ears, which should be considered in the selection and fitting of spectacles.
Accommodation
Accommodation is also affected in DS children with a lower amplitude and greater lag observed. A lag of more than +1.00D in more than half of children with DS was observed at approximately 25cm. Accommodative deficits in DS occur even without significant refractive error. Alongside these clinical signs, lens biometry reveals that individuals with DS have lenses that are thinner (3.27± 0.29mm vs 3.49±0.20mm) and of a lower refractive power (17.70±2.36D vs 19.48±1.24D) than controls. The Bifocals in DS (BiDS) study of participants aged 8-18 years old showed that a near addition improved near visual acuity and performance of certain literacy skills.
Visual acuity
Mean visual acuity is similar between infants under the age of two years with DS and without. Among 9-16 year olds, mean corrected acuity in DS (6/13) was less than controls (6/5). A study of adults with DS that, based on presenting visual acuity, visual impairment or blindness affected 43% of individuals. With refractive error corrected, the prevalence of visual impairment was effectively halved!
Strabismus
The prevalence of strabismus ranges from 19-34% in DS. Esotropia is significantly more prevalent than exotropia and vertical deviations. With the known high prevalence of hyperopia in children with DS, accommodative strabismus may be reduced by correcting refractive error. In 19% of cases, esotropia was corrected by spectacles. Among the few vertical deviations, congenital fourth nerve palsy was a common cause.
Amblyopia
Amblyopia is thought to affect 8.5-26% of individuals with DS. Patching or optical penalisation may be prioritised over atropine, as children with DS are suggested to be more susceptible to the cardiovascular side-effects of atropine.
Nystagmus
Nystagmus is estimated to affect 6-33% of individuals with DS. Manifest, fine, fast horizontal nystagmus appears frequently in DS and does not require intervention. A recent study reported that most cases of nystagmus were stable or resolved spontaneously. In this study, spasmus nutans was observed in one patient and neuroimaging implemented to exclude an intracranial lesion.
Lids and lashes
Characteristic eyelid features in DS include oblique palpebral fissures, epicanthal folds, and epiblepharon. Up to 50% of children with DS have blepharitis. The high proportion of blepharitis may be due to the narrow, slanted lid anatomy in combination with their increased susceptibility for infection. Infants with DS have been observed to have floppy eyelid syndrome also associated with papillary conjunctivitis and sleep apnoea.
Lacrimal system
Nasolacrimal duct obstruction and epiphora are reported to affect 17-36% and 15-32% of the population with DS, respectively. Anatomical anomalies in the lacrimal drainage in DS include punctal agenesis, canalicular atresia, canalicular stenosis and anteriorly-positioned inferior turbinate. Thus, obstruction of the lacrimal system in DS is complicated by abnormal anatomy as well as the failure of Hasner’s membrane to rupture.
Keratoconus
Prevalence estimates for keratoconus in DS range from 0.5-30%. The corneas of individuals with DS are inherently steeper, thinner and biomechanically weaker than non-DS counterparts. Given the clinical predisposition for keratoconus, it is conceivable that there may be a genetic link between keratoconus and chromosome 21. Others have attributed keratoconus in this group to mechanical trauma from eye rubbing, however, evidence for this is yet to be fully established.
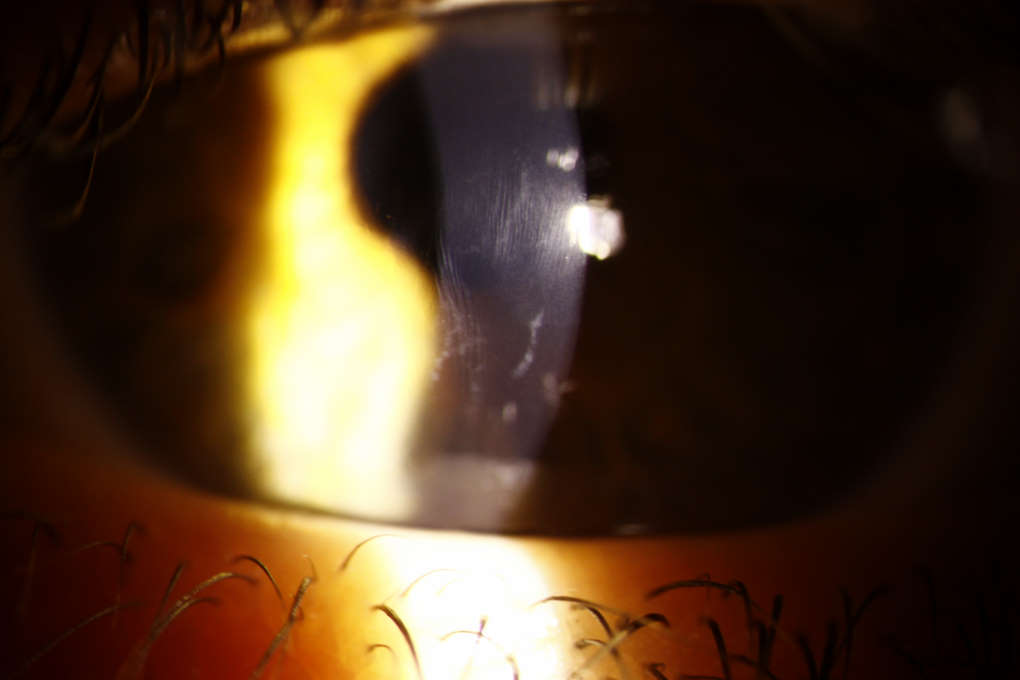
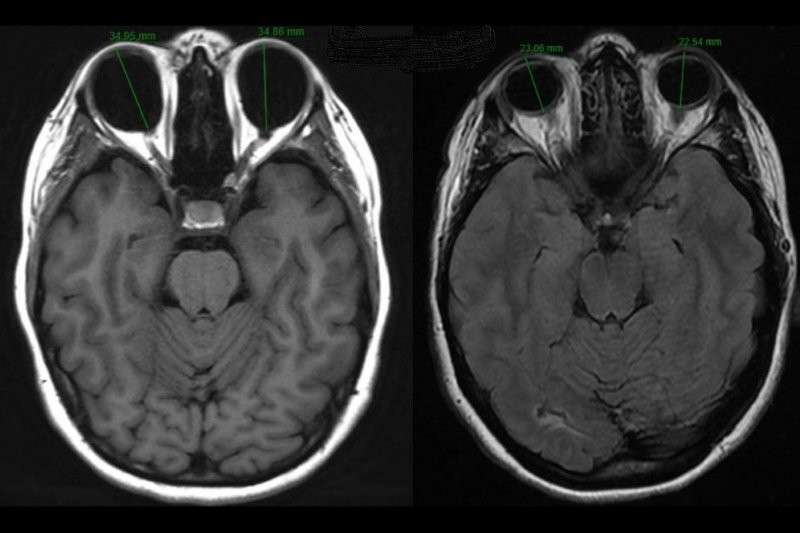
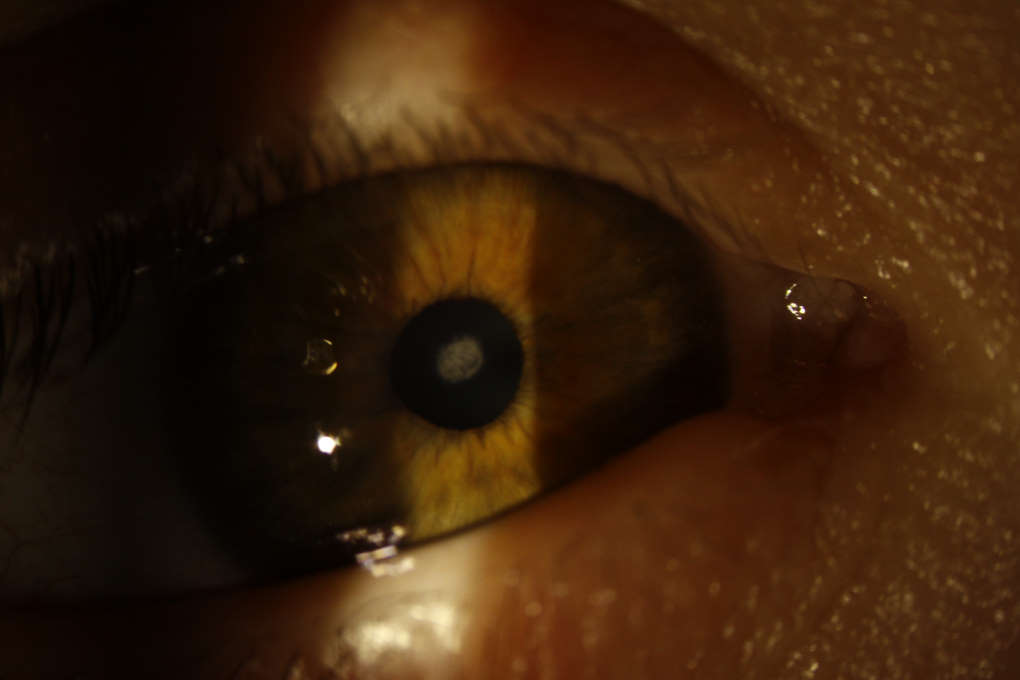
Vogt’s striae and anterior stromal scarring in an individual with DS and keratoconus
Brushfield spots
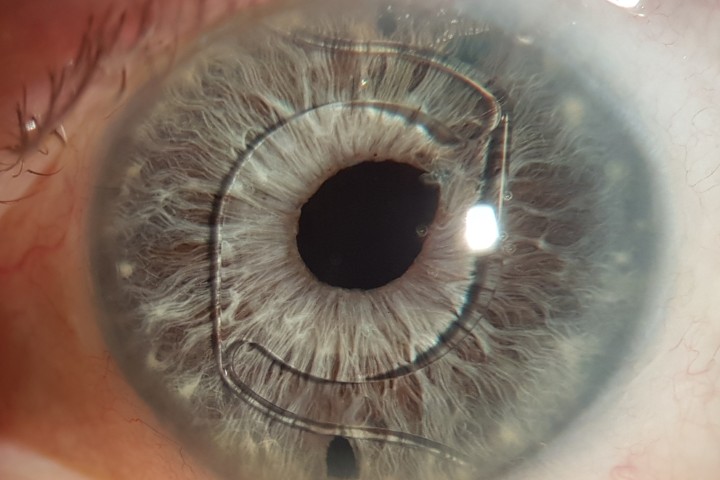
Spots of iris stromal hyperplasia, known as Brushfield spots, occurs in approximately 52% of individuals with DS with no functional consequence.
Cataract
A review by Creavin et al. showed that cataract occurs in 6-37% of children with DS in 10 out of 18 included studies. A prevalence of 5% or less was detected in the remaining eight studies. It has also been observed that age-related cataract is diagnosed at a significantly younger age in adults with DS than those without.
Congenital cataract in DS.
Optic nerve
Optic nerve elevation has been shown to affect approximately 3% of the DS population. Disc pallor affects 1-5% of individuals in this group. A 10-year retrospective review reported additional features of the optic nerve head including myopic appearance, peripapillary atrophy, peripapillary pigmentation and disc drusen. Of eight studies identifying glaucoma in children with DS, most indicated a prevalence of 1%. Two reported a higher prevalence of 5% and 7%. Glaucoma cases reported in the latter study included infantile glaucoma and glaucoma secondary to uveitis.
Retina
The reported prevalence of retinal abnormalities ranges from approximately 10-40%. Prevalence reports of an increased number (18) of retinal vessels crossing the optic nerve margin ranged from 13-38% in DS. Other retinal findings (occurring in 3% or less of the DS population) include myopic chorioretinitis, coloboma, embolism, focal pigment hypoplasia, retinal detachment, haemorrhage, vessel tortuosity, macular hypoplasia and congenital stationary night blindness. Approximately 17 cases of retinoblastoma in DS have been reported in the literature. It has been proposed that alterations to chromosome 21 may increase the susceptibility to retinoblastoma.
Ocular infection
A 10-year retrospective review revealed that the main causative micro-organism in microbial keratitis in DS was Streptococcus pneumoniae. Ophthalmic associations were lid disease (94%), atopic keratoconjunctivitis (44%), keratoplasty (33%) and use of topical corticosteroid (33%). A 2014 study of keratoplasty in 29 patients with intellectual disability reported one case of endophthalmitis.
Therapeutics
The New Zealand Formulary indicates that muscarinic antagonists, which are routinely used in ophthalmic examinations, should be used with caution in children with DS due to increased risk of toxicity. While no adverse reactions were reported in studies that performed cycloplegic refractions on children with DS, care should be taken to occlude the puncta for two minutes to minimise systemic absorption. Additionally, when selecting from ocular anti-hypertensives, those with cardiovascular and respiratory side effects should be avoided.
Ministry of Health recommendations for individuals with DS
Recommendations for ophthalmic care in DS indicate that between birth and one month, the red reflex test should be conducted and infants referred immediately to an ophthalmologist if cataract, strabismus or nystagmus is suspected. Otherwise, an initial ophthalmological assessment should be undertaken by nine months. The next review should occur between 18-24 months old and again at 4.5 years. Between the ages 5–13 years, assessments should be undertaken every two years. At 10 years the child may be referred to an optometrist for assessments every two years thereafter.
Conclusion
There are a variety of ophthalmic manifestations in this complex-needs group. Regular clinical review and further research into the visual impact of DS, with potential improvements in management and treatment options, could greatly benefit this disadvantaged population.
Additional reading
- Watt T, Robertson K, Jacobs RJ. Refractive error, binocular vision and accommodation of children with down syndrome. Clinical and experimental Optometry. 2015;98(1):3-11.
- Haugen OH, Høvding G, Eide GE. Biometric measurements of the eyes in teenagers and young adults with down syndrome. Acta Ophthalmol. 2001;79(6):616-625.
- Little J, Woodhouse JM, Saunders KJ. Corneal power and astigmatism in down syndrome. Optom Vis Sci. 2009;86(6):748-754.
- Cregg M, Woodhouse JM, Stewart RE, et al. Development of refractive error and strabismus in children with down syndrome. Invest Ophthalmol Vis Sci. 2003;44(3):1023-1030.
- Fong AH, Shum J, Ng AL, Li KK, McGhee S, Wong D. Prevalence of ocular abnormalities in adults with down syndrome in Hong Kong. Br J Ophthalmol. 2013;97(4):423-428.
- Krinsky-McHale SJ, Jenkins EC, Zigman WB, Silverman W. Ophthalmic disorders in adults with down syndrome. Current gerontology and geriatrics research. 2012;2012.
- Cregg M, Woodhouse JM, Pakeman VH, et al. Accommodation and refractive error in children with down syndrome: Cross-sectional and longitudinal studies. Invest Ophthalmol Vis Sci. 2001;42(1):55-63.
- Nandakumar K, Evans MA, Briand K, Leat SJ. Bifocals in down syndrome study (BiDS): Analysis of video recorded sessions of literacy and visual perceptual skills. Clinical and Experimental Optometry. 2011;94(6):575-585.
- Creavin AL, Brown RD. Ophthalmic abnormalities in children with down syndrome. J Pediatr Ophthalmol Strabismus. 2009;46(2):76-82.
About the author
Joyce Mathan completed a Bachelor of Optometry, First Class Honours, at the University of Auckland in November 2017. She is a doctoral candidate in the Department of Ophthalmology, NZ National Eye Centre and is supervised by Professors Charles McGhee, Dipika Patel, Dr Akilesh Gokul and Dr Samantha Simkin.