Ozurdex: one year on
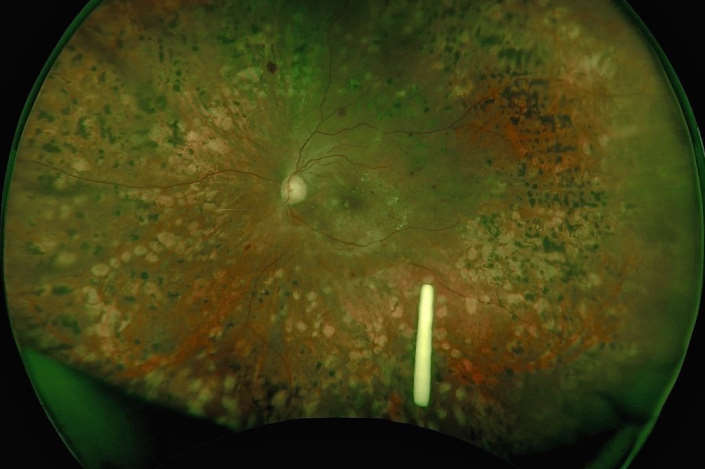
It has been just over a year since Ozurdex, a dexamethasone-sustained release implant, was first funded by Pharmac for diabetic macular oedema (DMO), the most common cause of visual loss in our working-age population.
The prescribing criteria dictated by Pharmac are as a second line treatment for DMO when it is still progressing after three injections of bevacizumab (Avastin) or where vascular endothelial growth factor (VEGF) inhibitors are unsuitable or contraindicated. The funding is restricted to pseudophakic patients with reduced visual acuity of between 6/9 and 6/48 with functional awareness of a reduction in vision. Under these criteria, the dexamethasone implants (Ozurdex) are to be administered no more than once every four months, up to a maximum of three implants a year.
The proven efficacy of intravitreal anti-VEGF agents makes them the first-line treatment, superseding macular laser, for centre-involving DMO. We also can now substitute bevacizumab for aflibercept (Eylea) in poor responders. Corticosteroids, however, are powerful non-specific, anti-inflammatory agents and have been reported to antagonize the action of VEGF.
Intravitreal dexamethasone implants for DMO do improve visual acuity and reduce central macular thickness, though in phase III trials more than 60% of patients had progression of cataract, so use is restricted to pseudophakic eyes. However, this would not preclude us using the implant straight after cataract surgery, which is a simple and effective option with Ozurdex shown to have reduced macular oedema after surgery.
Ozurdex is a more expensive medication to use compared to off-label triamcinolone. But it has the advantages of fewer injections and a better safety profile for the patient. Raised intraocular pressure (IOP) and glaucoma are a problem with all intraocular steroid use. After Ozurdex, there is a rise in IOP in about 30% of patients with peak pressure incidence at 60 days, but most pressure rises are resolved by 180 days and the trabeculectomy rate is less than 1% in trials. When we compare the raised IOP with intravitreal triamcinolone 4mg, however, the rise in pressure with triamcinolone was up to 68% with up to 5.9% requiring trabeculectomy. Dexamethasone is four times more potent than triamcinolone, but it is less lipophilic so does not accumulate in the lens and tabular meshwork as much. These two steroids also activate different patterns of gene expression in human trabecular meshwork cell lines, which may account for Ozurdex’s better safety profile.
Situations where Ozurdex may be used as first-line treatment would be after an arterial thromboembolic event or when a woman has not completed their family. I would also consider Ozurdex earlier in vitrectomised eyes where the stable pharmacokinetics of Ozurdex would be an advantage - although the pharmacy guidelines need to be followed closely to get funding.
Pharmac’s criteria restrict use to 6/48, but most trials include a vision of 6/60 at least. In practice, the limitations for the funded use of this drug do narrow the number that could benefit, however, off-label triamcinolone can be used here.
The use of steroids in diabetic macular oedema is well known. What is not fully understood is why some people respond to VEGF so well and others don’t. We see patients who have a very poor response to VEGF but a very good response to intravitreal steroids. This is likely explained by the broader effect on the anti-inflammatory cascade of steroid. Pathways that involve blood vessel endothelium, for example, may be more active in some DMO patients resulting in more oedema.
We know diffuse diabetic maculopathy still has poor outcomes. Can we identify those patients who will respond better to steroids than VEGF at the outset? We still use triamcinolone and triesence in phakic patients, however the side effect profile may be inferior. However, when we remove the lens we are able to use Ozurdex. Steroids may also be a better earlier option when there is sight-threatening exudate and hyper-reflective foci, as evidence suggests the exudate may resolve faster after steroid rather than VEGF.
We would also like to use fewer injections on our patients. Head-to-head studies showed Ozurdex allowed for at least three times fewer injections than VEGF. Mark Gillies’ Avastin patients in his BEVODEX Study received a mean of 8.8 injections vs 2.8 for Ozurdex, and we do see this in the real world.
Concluding comments
Better diabetic, lipid and blood pressure control are a primary focus but when these are not optimal we will still see patients with sight-threatening oedema, so having Ozurdex is a useful addition to our armamentarium. In the future, I would like to see Ozurdex’s indication extended for vein occlusions where funded options are currently very limited in New Zealand. Interestingly, Ozurdex is now available and funded in Australia for this indication.
References
- Dexamethasone intravitreal implant in previously treated patients with diabetic macular oedema: subgroup analysis of the MEAD study. Augustin et al. BMC Ophthalmology (2015) 15:150
- Dexamethasone intravitreal implant for treatment of diabetic macular oedema in vitrectomised patients. Boyer et al. Retina, 31;915-923, 2011
- Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular oedema. Boyer et al. Ophthalmology, Vol 121, 10, Oct 2014
- Effect of intravitreal dexamethasone implant on diabetic macular oedema after cataract surgery. Calvo et al. Retina 0:1–7, 2017
- Early response to ranibizumab predictive of functional outcome after dexamethasone for unresponsive diabetic macular oedema. Cinicnelli et al. BJO Online, April 21 2017, 10.1136/bjophthalmol-2017-310242
- Bevacizumab or dexamethasone implants for DME: two-year results (The BEVORDEX Study). Fraser-Bell et al. Ophthalmology, June 2016, Vol 123, 6, 1399-1401
- Perspective on the role of Ozurdex (dexamethasone intravitreal implant) in the management of diabetic macular oedema. Hemal Mehta, Mark Gillies and Samantha Fraser-Bell. Ther Adv Chronic Dis. 2015 Sep; 6(5): 234–245.
Drs Andrew Riley and Mark Donaldson were both involved in the phase III trial for Ozurdex in diabetic macular oedema and retinal vine occlusion. Both work at Eye doctors and Auckland Hospital and have a special interest in medical retina.