Stem cell trial for dry AMD on cards
The first clinical trial of a stem cell-based therapy for age-related macular degeneration (AMD) in humans could soon be reality, after US researchers used patient-specific stem cell-based therapy
To prevent blindness in animal models of geographic atrophy, the advanced “dry” form of AMD.
The protocols established by the animal study at the National Eye Institute (NEI), set the stage for a first-in-human clinical trial testing the therapy in people with geographic atrophy, for which there is currently no treatment.
"If the clinical trial moves forward, it would be the first ever to test a stem cell-based therapy derived from induced pluripotent stem cells (iPSC) for treating a disease," said Dr Kapil Bharti, who heads the NEI Ocular and Stem Cell Translational Research Unit.
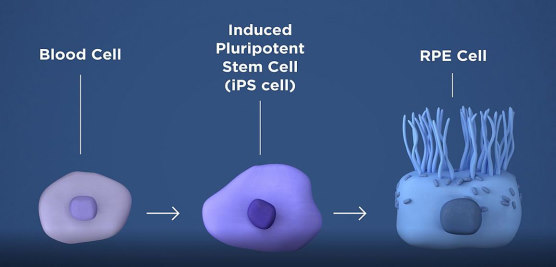
The therapy involves taking a patient’s blood cells and, in a lab, converting them into iPS cells, which can become any type of cell in the body. The iPS cells are then programmed by the researchers to become retinal pigment epithelial (RPE) cells which nurture photoreceptors, the light-sensing cells in the retina, but which die early in geographic atrophy. Once the RPE cells die, photoreceptors eventually also die, resulting in blindness. The therapy is an attempt to shore up the health of remaining photoreceptors by replacing dying RPE with iPSC-derived RPE.
Before they are transplanted, the iPSC-derived RPE are grown in tiny sheets one cell thick, on a biodegradable scaffold designed to promote the integration of the cells within the retina. A specially designed surgical tool was built for the task of inserting the patch of cells between the RPE and the photoreceptors.
In the STM paper, Bharti describes testing the approach in rat and pig models. Ten weeks after the human iPSC-derived RPE patches were implanted in the animals’ retinas, imaging studies confirmed that the lab-made cells had integrated within the animal retina and functioned properly.
Further tests showed that the transplanted RPE cells were pruning photoreceptors via phagocytosis, another RPE function that helps keep photoreceptors healthy. In addition, electrical responses recorded from photoreceptors rescued by RPE patches were normal; whereas photoreceptors treated with a control empty scaffold had died.
The animal studies inform the processes for conducting a first-in-human clinical trial testing the treatment. "Adhering to the protocols helps ensure that the transplanted cells function reliably, and that unintended consequences are minimised," Bharti said.
The planning of a Phase I clinical trial testing the safety of the iPSC-based therapy for geographic atrophy is now underway and will be initiated after US Food and Drug Administration approval.