The potential of ROCK inhibitors in the treatment of ocular disease
Rho-associated coiled-coil protein kinase (ROCK) inhibitors, have shown significant therapeutic potential for a wide range of ocular and non-ocular diseases. This includes new treatments for corneal endothelial disease, glaucoma, cardiovascular disease (Fasudil), neuronal disease, certain types of cancer, pulmonary diseases, erectile dysfunction and gastrointestinal diseases.
Rho-kinases are one of the most studied kinases since their discovery in the mid 1990s, with almost 160 patent applications in the past 20 years. In humans, there are two highly-conserved isoforms, ROCK1 and ROCK2, with overlapping and compensatory function. They are grouped within the AGC family of serine/threonine kinases. Both isoforms are ubiquitously expressed in the human body, including most eye tissue except the lens. ROCK regulates a number of key cellular pathways that influence actomyosin contraction, cell adhesion, cytoskeletal organisation, cell cycle progression, metabolism and apoptosis.
As it is postulated that some disease processes are initiated by an overexpression of ROCK-mediated pathways, it has become an emerging target for treatment of disease. ROCK inhibitor acts on ROCK by competitively binding to its ATP-binding site, thereby influencing the outcomes of downstream ROCK-mediated cellular pathways.
ROCK inhibitors have wide-ranging effects in the human body when administered systemically, including a rapid and pronounced drop in blood pressure and reversible decrease in lymphocyte count. Development of therapies needs to overcome these potential adverse effects. ROCK2 specific inhibitors and soft inhibitors are being developed to mitigate systemic side effects. Topical use, such as in ophthalmology, partly bypasses these systemic limitations and has really taken off. This article summarises some of the notable current and future uses of ROCK inhibitor in the treatment of ocular disease.
Glaucoma
ROCK inhibitors constitute a potential new class of glaucoma and ocular hypertension medication targeting the conventional pathway of aqueous humour drainage via the trabecular meshwork and Schlemm’s canal, which carries 80-90% of aqueous humour out of the eye. In contrast, most glaucoma medications only target the uveoscleral pathway (prostaglandins and α2-agonists) or aqueous humour production (ß-blockers, carbonic anhydrase inhibitors, and α2-agonists). ROCK inhibitors relax the trabecular meshwork and Schlemm’s canal cells, reverse myofibroblastic changes, widen Schlemm’s canal, increase permeability and reduce outflow resistance.
Laboratory studies have shown that cultured trabecular meshwork cells and Schlemm’s canal endothelial cells treated with ROCK inhibitor undergo reversible changes including decreased actin stress fibres, focal adhesions and intercellular interactions. In addition, aqueous humour of glaucoma patients contain increased amounts of bioactive factors such as lysophosphatidic acid and TGF-ß2 that could cause cells to become myofibroblasts. ROCK inhibitor Y-27632 was able to suppress this fibrogenic activity in cultured trabecular meshwork cells and prevent their transition into myofibroblasts when they were exposed to these bioactive factors. These results suggest a central role of ROCK signalling in the pathogenesis of glaucoma.
The overall effect of ROCK inhibitor Y-27632 application by topical application or intracameral injection in animals is a dose-dependent increase in aqueous humour outflow and reduction in IOP. These are facilitated by trabecular meshwork relaxation, Schlemm’s canal widening, increases in giant vacuoles in Schlemm’s canal endothelial cells, expansion of juxtacanalicular tissue, relaxation of ciliary muscles and wash-out of extracellular matrix in the conventional pathway.
IOP-lowering in animals has also been demonstrated after administration of other ROCK inhibitors, including Y-39983, fasudil (HA-1077), H1152, netarsudil (AR-13324), and SR-367. Netarsudil also decreased episcleral vein pressure in rabbits. Some ROCK inhibitors also exhibited neuroprotective effects by promoting axon outgrowth and retinal ganglion cell survival in a number of animal models. Inhibition of the transformation of tenon fibroblasts into myofibroblasts, and improved success rate was evident in rabbits after receiving glaucoma filtration surgery. ROCK inhibitors could therefore potentially be developed into anti-scarring agents after filtration therapy.
Several ROCK inhibitors including Y-39983 (SNJ-1656), fasudil, AMA0076, AR-12286, and netarsudil (AR-13324) are being evaluated for their safety and efficacy for glaucoma and ocular hypertension in clinical trials. All produced hypotensive effects in healthy people.
The only ROCK inhibitor that has been approved for clinical treatment of ocular hypertension and glaucoma is ripasudil (K-155) and only in Japan. In a multicentre, prospective, open-label study in Japan, the effect of ripasudil 0.4% in patients with pseudoexfoliation glaucoma, primary open angle glaucoma, or ocular hypertension was analysed.
When applied twice daily as monotherapy in 174 patients, the mean IOP reduction at 52 weeks compared to baseline at trough (before instillation) and peak (two hours after instillation) were −2.6 and −3.7 mmHg, respectively. As additive therapy (181 patients), the respective IOP reductions were −1.4 and −2.4 mmHg when combined with prostaglandin analogues, −2.2 and −3.0 mmHg with β‐blockers, and -1.7 and −1.7 mmHg with fixed combination drugs. When the 52 weeks was divided into two halves, additional IOP-lowering effects of ripasudil in the second half of the study were demonstrated (Fig 1). Eyes with baseline IOP >21 mmHg showed the greatest reduction in the second half. This long-term effect suggests that the ROCK inhibitor could have slowly remodelled the extracellular matrix of the trabecular meshwork and Schlemm’s canal, eliciting mild and delayed reduction in IOP. ROCK inhibitors may also restore blood flow around the optic disc, thereby slowing the progression of glaucomatous optic neuropathy, but further studies are required to investigate this.
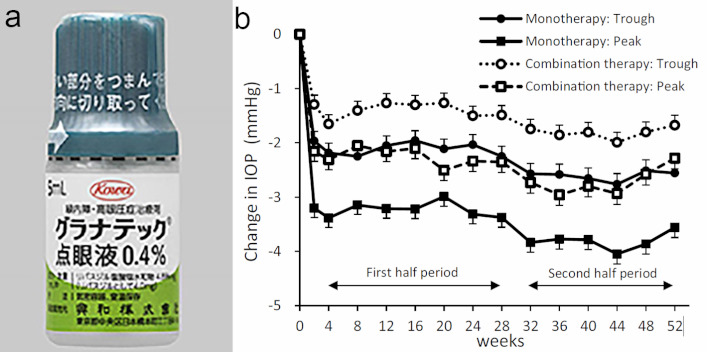
Fig 1. (a) A bottle of Ripasudil (Japan) and (b) changes in IOP by 0.4% ripasudil. Changes in IOP before instillation (trough) or at 2h after instillation (peak) in monotherapy and combination therapy groups are shown
Due to its vasodilatory effects, conjunctival hyperaemia is a common side effect of topical ROCK inhibitor. As expected, conjunctival hyperaemia was reported in 74.6% of patients in the previous study. This ‘cosmetic’ side effect occurs rapidly after instillation but subsides relatively quickly. Interestingly, this side effect did not cause major problems in reducing adherence or satisfaction, and most cases were mild, transient and resolved spontaneously. Other side effects such as blepharitis (20.6%) and allergic conjunctivitis (17.2%) did cause 14.4% of patients to withdraw from the study. These will be pitfalls to watch out for when considering the application of ROCK inhibitors in the future.
Soft ROCK inhibitors that are more prone to degradation, such as AMA0076 and its modifications, have been under investigation. These drugs appear to be able to induce significant IOP lowering without significant hyperaemia in humans and animals.
Corneal endothelial disease
ROCK inhibitor has shown great promise in the treatment of corneal endothelial disease. It is well known that corneal endothelial cells lack proliferative potential in vivo. For patients with corneal endothelial disease, progressive loss of endothelial cells leads to stromal oedema and loss of visual acuity. The only sight-saving treatment available is a corneal donor transplant, which is limited by a growing corneal donor shortage.
Scientists have been working on developing alternative treatments for corneal endothelial disease. This includes bioengineering corneal endothelial cells in vitro, using both primary corneal endothelial and stem cell lines. In vitro studies have shown that the selective ROCK inhibitor-Y27632 improves primary corneal endothelial cell culture by aiding cell attachment, enabling retention of mature corneal endothelial cell morphology and enhancing cell yield. Recent work with embryonic, induced pluripotent and adult stem cells (including Transition Zone Stem cells) have shown that ROCK inhibitor also acts to enhance cell proliferation and differentiation.
In vivo animal studies have further shown that administration of ROCK inhibitor into eyes with damaged corneal endothelium facilitated corneal endothelial wound healing. For example, in mechanically scraped rabbit and primate corneal endothelium wound models, ROCK inhibitor injections into the anterior chamber were shown to restore corneal transparency after three months, while in control eyes the cornea remained oedematous.
Clinical trials using ROCK inhibitor have so far consisted of small pilot studies. A study of 11 patients with bullous keratopathy, trialled a single injection of cultured, corneal endothelial cells from a human donor into the anterior chamber of the affected eye. The donor cells were cultured in vitro in a medium supplemented with ROCK inhibitor. Patients were placed in the prone position for three hours after surgery to allow the cells to adhere to the posterior aspect of the cornea. After a 24-week period, restoration of corneal transparency, reduction in corneal thickness and improvement in visual acuity was observed. Patients were followed-up over a two year period and were shown to have no significant ocular or systemic side effects as a result of the treatment, suggesting that it may be feasible to use this technique in a larger trial. Issues with this approach include inconsistency in achieving a sufficient cell density with injection and the consequences of injecting cellular particles into the anterior chamber.
An alternative therapeutic approach that has been discussed in the literature is the culturing of corneal endothelial cells in vitro with ROCK inhibitor and growing the cells on an artificial Descemet’s membrane or cell carrier. This could then be used as a partial-thickness graft for corneal transplantation, using the standard DMEK procedure currently used to insert a donor graft into eyes with corneal endothelial disease.
Larger and lengthier randomised-controlled trials are required to make any inferences about the efficacy of ROCK inhibitor in treatment. Despite the challenges that remain, the positive effect ROCK inhibitor has on corneal endothelial cell growth, proliferation and wound healing suggest that they have significant potential in providing an alternative treatment for patients with corneal endothelial diseases.
Other diseases
ROCK inhibitors also seem to possess anti-inflammatory properties by inhibiting cell migration, invasion, and cytokinesis. Topical ripasudil was able to reduce aqueous flare in glaucoma patients with anterior uveitis. In a rat model of endotoxin-induced uveitis, systemic ripasudil significantly reduced infiltrating cells and protein exudation in the aqueous humour and the iris-ciliary body and reduced adherent leukocytes in retinal vessels.
Summary
In summary, ROCK inhibitors represent an exciting new class of drugs with demonstrated efficacy in treating glaucoma, with possible applications in corneal endothelial and inflammatory eye diseases. Ripasudil (generic name Glanatec) is not available in New Zealand yet and is quite expensive at approximately US$70/5ml. We hope to see further development of ROCK inhibitors with reduced side effects and reduced costs in the future.
About the authors
Hannah Ng is a final-year medical student at the University of Auckland. She has an interest in ophthalmology and has recently completed a BMedSc (Hons) project with the Department of Ophthalmology, New Zealand National Eye Centre. In 2012, Hannah was awarded the Prime Minister's Future Scientist Prize and is now on the selection panel for the award.
Dr Jie Zhang is a research fellow in the Department of Ophthalmology, University of Auckland. Jie is using her biomedical science background to devise treatments for ophthalmic conditions such as biocompatible scaffolds for corneal regeneration, and stem cell treatments for corneal endothelial diseases.
References
- Defert O, Boland S. Rho kinase inhibitors: a patent review (2014 - 2016). Expert Opin Ther Pat 2017;27:507-515.
- Narumiya S, Ishizaki T, Uehata M. Use and properties of ROCK-specific inhibitor Y-27632. Methods in enzymology 2000;325:273-284.
- Honjo M, Tanihara H, Inatani M, et al. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci 2001;42:137-144.
- Honjo M, Tanihara H. Impact of the clinical use of ROCK inhibitor on the pathogenesis and treatment of glaucoma. Japanese journal of ophthalmology 2018;62:109-126.
- Tanihara H, Inoue T, Yamamoto T, et al. One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta ophthalmologica 2016;94:e26-34.
- Pipparelli A, Arsenijevic Y, Thuret G, Gain P, Nicolas M, Majo F. ROCK inhibitor enhances adhesion and wound healing of human corneal endothelial cells. PLoS One 2013;8:e62095.
- Okumura N, Koizumi N, Ueno M, et al. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. The American journal of pathology 2012;181:268-277.
8. Kinoshita S, Koizumi N, Ueno M, et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N Engl J Med 2018;378:995-1003.