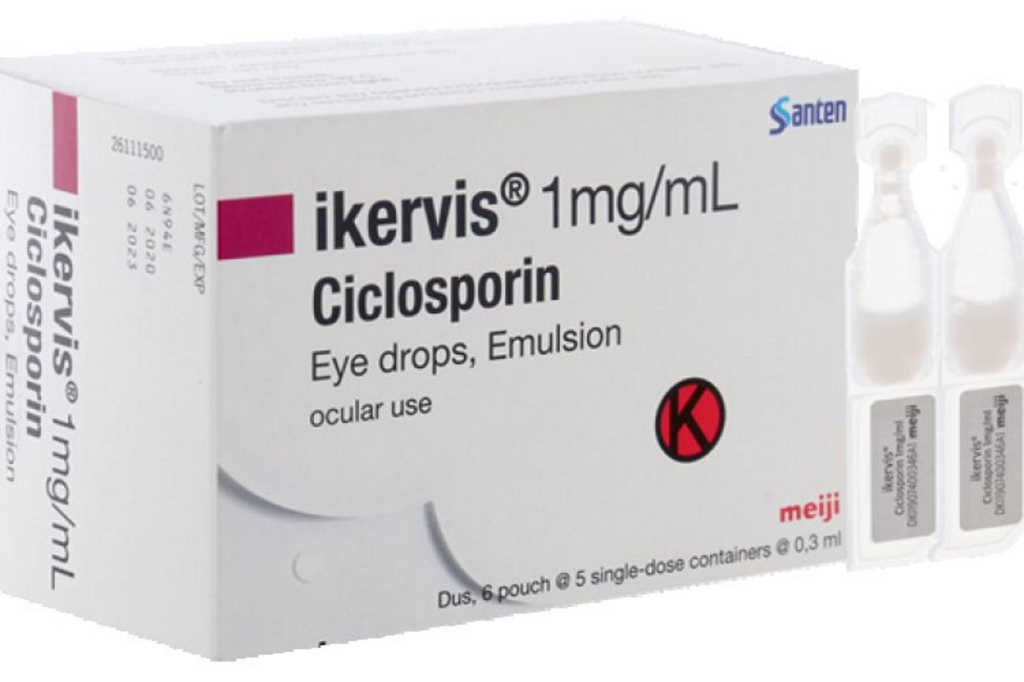
Ciclosporin gains public funding in Australia
Ciclosporin (Ikervis) has been listed on the Pharmaceutical Benefits Scheme (PBS) in Australia, saving around 7,500 severe keratitis patients with dry eye disease (DED) an estimated A$900 each per year, said Greg Hunt, Minister for Health and Aged Care.
Commenting on whether New Zealand’s ophthalmologists would welcome Pharmac taking the same tack, dry eye specialist Professor Jennifer Craig, from the Ocular Surface Laboratory at Auckland University, said that they would, but it’s complicated. “Pharmac would be unlikely to agree to fund it. I had to help fight to keep compounded cyclosporin funded here and they eventually agreed to it, relying on the fact that it’s relatively rarely prescribed. This is because it only has significant benefit to those with underlying inflammatory dry eye disease, rather than the more common evaporative form.”
Wider accessibility of the commercial form of cyclosporin runs the risk of clinicians prescribing it where it isn’t properly indicated, explained Prof Craig. “Because we don’t have many suitable treatments, it would tend to become the default – but not necessarily the appropriate – treatment. This would blow out Pharmac’s budget, if they could even be persuaded to fund it, and ultimately I think they would withdraw funding of cyclosporin entirely, which would be a tragedy for those who are truly dependent on it.”
Australia’s Ministry of Health also announced that the anti-VEGF treatment brolucizumab (Beovu) will also be PBS listed, saving around 12,800 Australians more than A$8,800 per year in wet age-related macular degeneration treatment.