New TED therapeutic?
May 3, 2022
Staff reporters
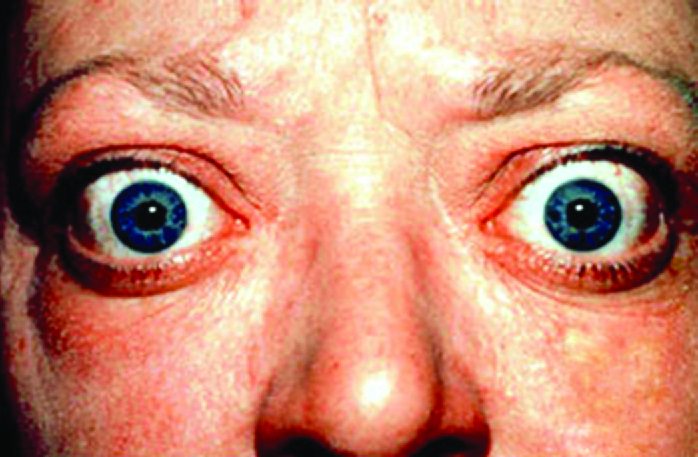
ValenzaBio’s monoclonal antibody drug to treat thyroid eye disease (TED) has been cleared by the US Food and Drug Administration to enter phase 1a clinical trials.
The drug, VB421, targets the insulin-like growth factor type 1 receptor (IGF-1R), which plays a central role in the pathogenesis of TED. “We know that an easy to administer, subcutaneous therapy would be a welcome treatment option for this devastating disease… Our phase 1a study will provide critical data that will inform dose selection and development plans for our future trials in TED patients,” said Dr Greg Keenan, ValenzaBio chief medical officer.
Enrolment of healthy volunteers for the trial is expected to begin in the second quarter of 2022.