Red-light therapy for myopia control
Hot on the heels of the official launch at the Asia-Pacific Academy of Ophthalmology Congress in Kuala Lumpur, Eyerising International’s new at-home, red-light therapy device for myopia management, Eyerising Myopia Management Device, attracted significant attention at CCLSNZ 2023.
Eyerising Myopia Management Device is an exciting addition to the myopia control tool kit, said Kiwi distributor, Ophthalmic Instrument Company’s (OIC’s) Tim Way. “The treatment is fuss free, home-based and non-invasive. It can be started when the child is just three years old; it’s a potential game changer.”
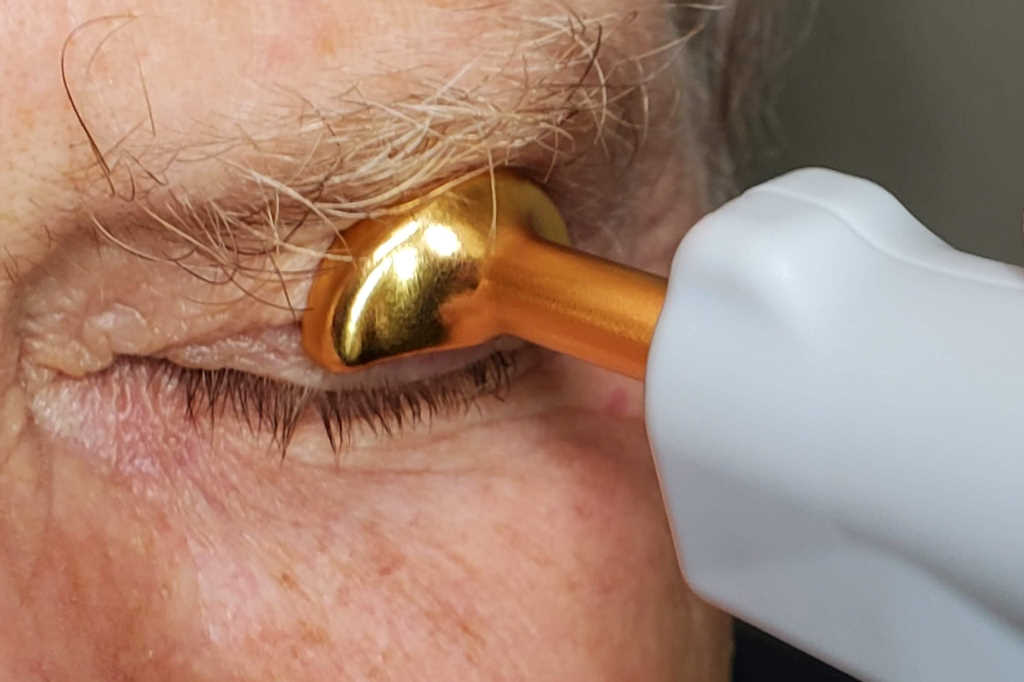
Repeated low-level red-light (RLRL) therapy is clinically proven to prevent or slow down the progression of myopia, said the Melbourne-based med-tech developer, adding in some cohorts it is achieving “up to 87.7% myopia control efficacy”. A retrospective analysis of a randomised controlled clinical trial published by Ophthalmology and Therapy found RLRL therapy to be effective in about a quarter of the population. The study examined 434 myopic children of Chinese ethnicity, aged 3-17 years, with a spherical equivalent refraction of at least -0.50 diopters, complete axial length (AL) data and no history of myopia control treatments. It found 26.5% of the children experienced AL shortening > 0.05mm per year following 12 months of RLRL therapy, with the greatest effect achieved in younger children with higher myopia. The authors recommended further experimental studies to achieve more effective targets for AL modulation, as the key molecular mechanisms of AL shortening from RLRL remain unexplained. A two-year post-trial follow-up study, published by Clinical and Experimental Ophthalmology, found the RLRL therapy had a sustained effect, noting only a modest rebound after treatment cessation.
Widely used and approved for amblyopia treatment in China for about a decade, Eyerising Myopia Management Device is now CE certified, registered with MedSafe in New Zealand and is currently under review by Australia’s Therapeutic Goods Administration. The device consists of semiconductor laser diodes, which deliver low-level red light with a wavelength of 650±10nm at an illuminance level of approximately 1,600 lux through the pupil to the fundus.
Priced at $120 per month*, Eyerising Myopia Management Device will be offered to patients through a subscription-based model, with OIC managing distribution, training and any servicing required. After a simple set up connecting the device to the patient’s Wi-Fi, treatment can start in the comfort of the child’s home, said Way. Two three-minute sessions per day are required, at least four hours apart. For ease of use, Eyerising recommends young patients complete a session in the morning before school and another in the afternoon or evening.
*Low-dose atropine (0.02%) costs about $140, plus postage, for three months’ worth.