Stargardt drug trial begins
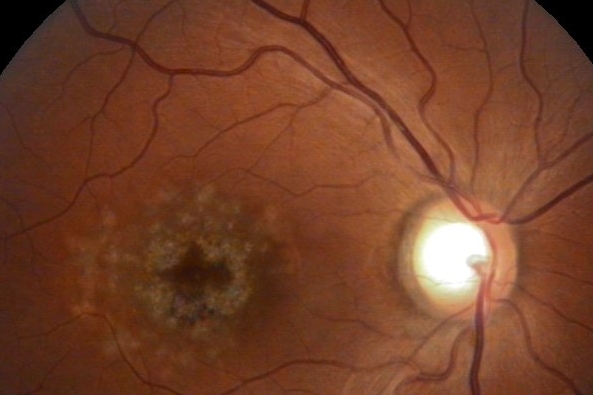
Belite Bio announced it has completed enrolment for its pivotal global phase 3 DRAGON trial of oral treatment Tinlarebant for patients with Stargardt disease.
Tinlarebant is a novel oral therapy intended to reduce the accumulation of toxins in the eye that cause Stargardt disease and contribute to geographic atrophy (GA) or advanced dry age-related macular degeneration.
The DRAGON trial includes 90 adolescent subjects across 11 countries, including Australia. It marks an important milestone for our late-stage programme in Stargardt disease, said Dr Tom Lin, CEO of Belite Bio. “Importantly, we are pleased to see the embrace of this much-needed therapeutic opportunity by the Stargardt patient community. DRAGON has potential to be the first global phase 3 clinical trial to demonstrate a treatment benefit in patients and we look forward to sharing the interim safety and efficacy data in mid-2024,” he said.
In 2022, 12-month interim data from the phase 2 study showed Tinlarebant halted or slowed lesion growth and was granted fast-track designation and rare paediatric disease designation in the US, plus orphan drug designation in both the US and Europe for Stargardt disease.
Topline data for Tinlarebant in the phase 2 Stargardt study are expected in the fourth quarter of 2023, with interim data for the phase 3 DRAGON trial expected by mid-2024. Belite also plans to have the first patient enrolled in its two-year phase 3 PHOENIX study of Tinlarebant for geographic atrophy in the third quarter of 2023.