Teprotumumab promising for TED
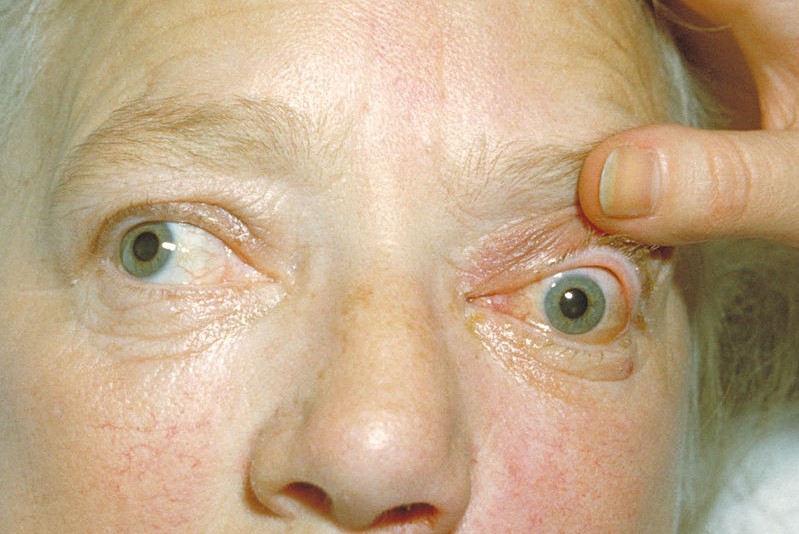
Horizon Therapeutics’ phase 3 clinical trial of teprotumumab, an investigational medicine for the treatment of active thyroid eye disease (TED), shows positive effects on diplopia (double vision), quality of life and clinical activity score (CAS).
“The results of this study are very encouraging, showing that 68% of patients had an improvement of at least one grade in double vision and improved on other measures including quality of life and CAS score,” said lead researcher Dr Raymond Douglas, Cedars Sinai Medical Centre, Los Angeles. “Thyroid eye disease commonly causes a variety of vision impairments, with double vision reported in about half of all people living with the disease, and almost 70% of patients enrolled in the study. People suffering from double vision often lose the ability to perform daily tasks, like reading and driving, impacting their ability to work and causing depression,” he said.
The data, presented at the American Society of Ophthalmic Plastic and Reconstructive Surgery (ASOPRS) symposium mid-October, showed an improvement from baseline of at least one grade in diplopia (68% compared to 29% of patients receiving placebo). This endpoint measured the percentage of patients who reported at least some diplopia at baseline in the study eye and who had a reduction of ≥ 1 grade with no corresponding deterioration (≥ 1 grade worsening) in the other eye at week 24.
Patients on teprotumumab demonstrated a mean change of 13.79 on Graves' Ophthalmopathy Quality of Life scale compared with a change of 4.43 for patients receiving placebo. Also, more participants achieved a CAS value of 0 or 1 with teprotumumab (59% vs 21% of placebo). The CAS scale is used to assess TED’s disease activity and measures the degree of inflammation, pain, swelling and redness. It ranges from zero to seven, with a zero-score representing no activity.
The Phase 3 trial builds on data presented earlier this year, demonstrating a significant benefit of teprotumumab on proptosis (bulging eyes). Teprotumumab is currently under review by the FDA.