AMD, DMO therapy research review
Emerging treatment options for geographic atrophy (GA) secondary to age-related macular degeneration
Hannah Khan
Clinical Ophthalmology, 2023
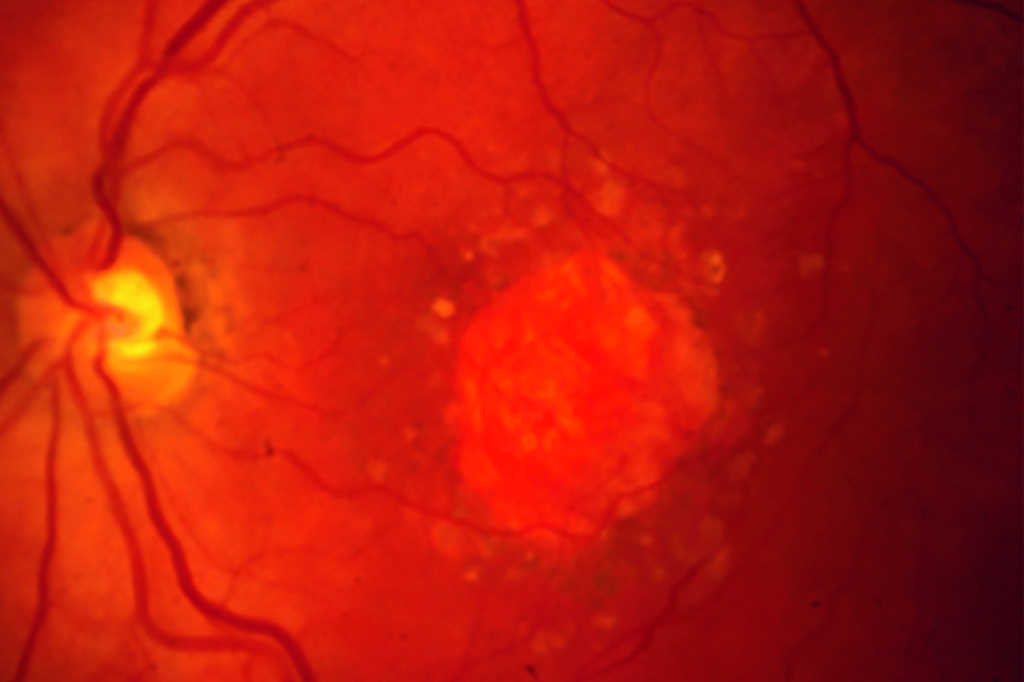
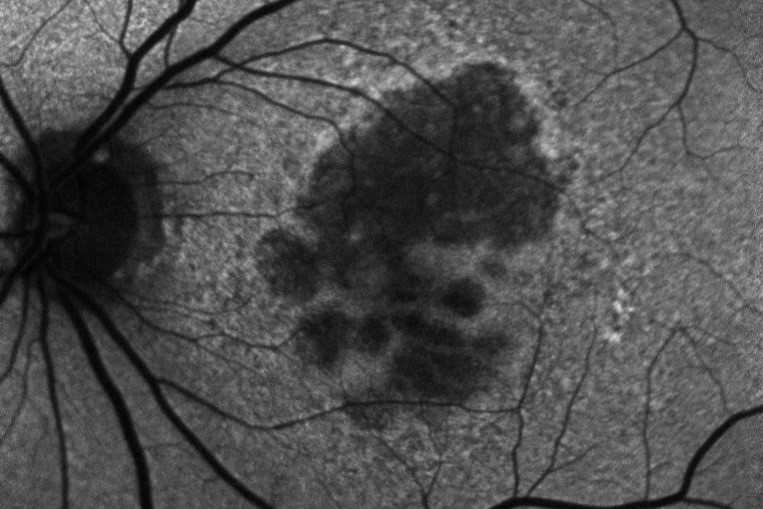
Review: This review evaluated the current status of treatment for GA, describing several different inhibitors of the complement cascade. Pegcetacoplan (APL-2) is a modified PEGylated peptide that binds to C3 and C3b, ultimately blocking all three complement system pathways. Filly phase 2 and now phase 3 trials Oaks and Derby are out for evaluation. At 24 months there is 18-22% reduced growth of geographic atrophy. Neuroprotective agents, ocular gene therapy and stem cell therapy are also being evaluated for future options.
Comment: Pegcetacoplan (Syfovre) is the first FDA-approved treatment for GA and an exciting development. This intravitreal injection is now approved for injection every 25-60 days. Phase 3 results showed a significant reduction in lesion size at 24 months for subfoveal and extrafoveal GA. However, rates of choroidal neovascularisation may increase from 3% in the control group to 7% in the monthly injection group, with no functional visual improvements noted.
Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regarding glycaemic control and body weight reduction
M Nauck et al
Cardiovascular Diabetology (2022) 21:169
Review: Tirzepatide was one of several single-molecule GIPR and GLP-1R agonists developed after the early work in multi-receptor peptides. Given as a weekly injection, it stimulates insulin secretion, reduces glucagon, delays gastric emptying, decreases food intake and reduces body weight.
From the phase 3 Surpass 1-5 trials, after 30 weeks 81%-93% of patients had an HBA1c less than 52mmol/mol and bodyweight was reduced by 5.4-11.7kg. The higher the initial body weight, the better the response. In Surpass trials, 38% of patients achieved HbA1c <41mmol/mol, a level considered non-diabetic. Nausea (18%) and, less commonly, vomiting were the main side effects reported.
Comment: We are reaching a new era in type 2 diabetes treatment and weight loss. Since New Zealand funded dulaglutide (Trulicity), a GLP-1 agonist, we are seeing weight loss and improved diabetic control. With the arrival of even more effective peptides such as semaglutide (Ozempic) and tirzepatide (Mounjaro), we are going to see patients with rapid improvements in diabetic control and weight loss. The future in this field is exciting. However, a word of caution: rapid reduction in HbA1c may cause rapid progression of diabetic retinopathy, which we will all need to be ready for. Further studies are looking at the optimal rate of dosing and HbA1c reduction.
Aflibercept versus faricimab in the treatment of nAMD and DMO: a review
Sławomir Liberski et al
Int. J. Mol. Sci. 2022, 23, 9424.
Review: Faricimab is the first bispecific monoclonal antibody for intravitreal use that can neutralise vascular endothelial growth factor-A and angiopoeitin-2. Due to its prolonged activity, faricimab pushed out the interval required between successive injections to three to four months in neovascular age-related macular degeneration (nAMD) and diabetic macular oedema (DMO) patients in phase 3 trials. To compare the effects of faricimab and aflibercept treatment in DMO patients, two randomised, multicentre studies, Yosemite and Rhine, showed faricimab injections every 16 weeks was as effective on vision and retinal anatomy as eight-weekly aflibercept.
Faricimab was also compared with aflibercept in the phase 3 Tenaya and Lucerne clinical trials for nAMD. During the first year of treatment, 80% of patients in the Tenaya study and 78% of patients in the Lucerne study could receive injections less frequently than every two months, as used in the aflibercept regimen.
Comment: This treatment represents a step forward by reducing the treatment burden and the current list price is very favourable. With the likely cost-effectiveness of this new treatment, we hope to see Pharmac funding expedited. Due to faricimab having a dual mode of action, we may also see a difference in patients not responding to currently available therapy.

Dr Andrew Riley is a consultant ophthalmologist at Waitakere Hospital, Greenlane Clinical Centre and Eye Doctors in Ascot and Ormiston hospitals.