ARVO 2021 highlights
Due to the ongoing Covid-19 pandemic, the Association for Research in Vision and Ophthalmology (ARVO) held its annual meeting virtually from 1-7 May. The following are a few selected highlights:
Three-monthly AMD treatment
Professor Robyn Guymer, University of Melbourne, presented phase 3 trial results for faricimab, the first bispecific antibody designed for intraocular use in treating neovascular age-related macular degeneration (nAMD). The results showed that with 12-week dosing intervals, faricimab met its primary efficacy endpoints of non-inferiority to aflibercept in change in the best-corrected visual acuity, durability and safety. The drug, Dr Guymer explained, may synergistically promote vascular stability and reduce treatment burden through extended durability in patients with nAMD.
VA test for non-communicative patients
Auckland’s Objective Acuity and Dr Christina Ohnsman, an ophthalmologist in Pennsylvania, USA, conducted a pilot study of a novel visual acuity (VA) test for children with CLN2 disease, a form of Batten disease that causes a rapid loss of vision after the child has lost cognitive, motor and language abilities. Traditional vision tests requiring input from the patient cannot be used to measure the extent of vision loss, resulting in lack of natural history data on the range, severity and impact of visual impairment. Researchers evaluated a novel vision test based on an involuntary eye movement reflex that needs no input from the child. The ‘threshold visual acuity test’ proved useful in measuring VA in children with CLN2 disease and is a promising method of testing vision in other populations that are unable to communicate, they reported.
Glaucoma linked with foetal stress
A study led by Dr Kathleen Dyer, Lions Eye Institute, Western Australia, has shown that foetal head circumference (FHC) growth is strongly correlated with retinal nerve fibre layer (RNFL) thickness, the latter being an early marker for glaucoma. Adverse early life conditions and restricted FHC growth may predispose towards thinning of the RNFL in child and adult life and the long-term risk of glaucoma, researchers concluded.
Childhood glaucoma genes identified
A team led by Lachlan Knight, Flinders University, Adelaide, reviewed childhood glaucoma referrals to the Australian and New Zealand Registry of Advanced Glaucoma (ANZRAG) over a 13-year period. The team identified changes in 18 genes associated with childhood glaucoma and early-onset glaucoma. Almost one quarter of the families involved in the study received a genetic diagnosis, which helped with providing individuals and families with appropriate clinical diagnoses, management and genetic counselling. Primary glaucoma was most prevalent in both cohorts, with biallelic CYP1B1 variants reported in twice as many females as males.
AMD: gene therapy to oust IVT?
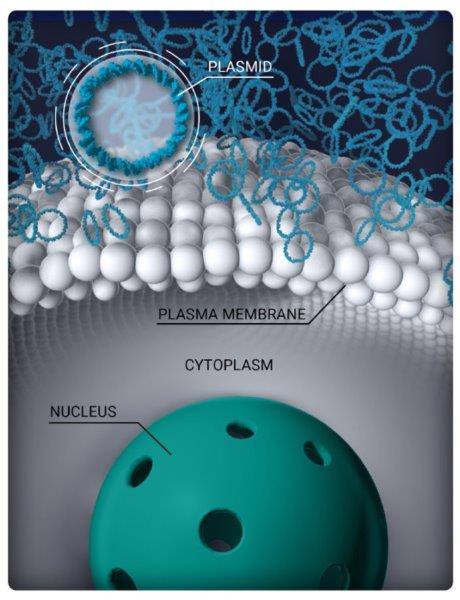

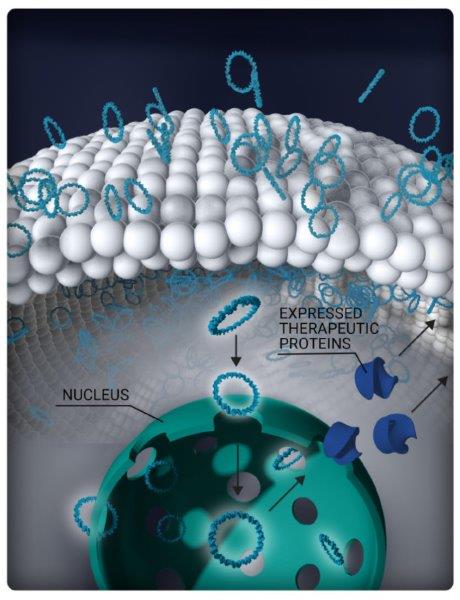
Eyevensys' EY809 before, during and following electronic pulse application
Eyevensys’ EYS809, a sustained drug-delivery vehicle for anti-VEGF, may replace repeated intravitreal injections and improve vision in patients with wet-AMD. The non-viral, dual-gene platform exploits electrotransfection of DNA plasmids to induce ciliary muscles to secrete aflibercept and decorin (see images above), said Dr Elise Orhan, the preclinical project leader at Eyevensys. “The combined effect of aflibercept and decorin is expected to provide benefits over aflibercept alone by reducing choroidal neovascularisation (CNV) leakage and subretinal fibrosis.”
Protein therapy alternative to anti-VEGF
Ocugen’s OCU200 is in preclinical development for the treatment of diabetic macular oedema, diabetic retinopathy and wet-AMD for the roughly 50% of patients who do not respond to anti-VEGF or corticosteroids. A study to evaluate the efficacy of the therapeutic protein demonstrated that it inhibited cell proliferation, cell invasion and tube formation by endothelial cells. In an oxygen-induced retinopathy mice model, it reduced avascular areas by 68%, which was comparable to the results of aflibercept treatment.