DMO drop trial progresses
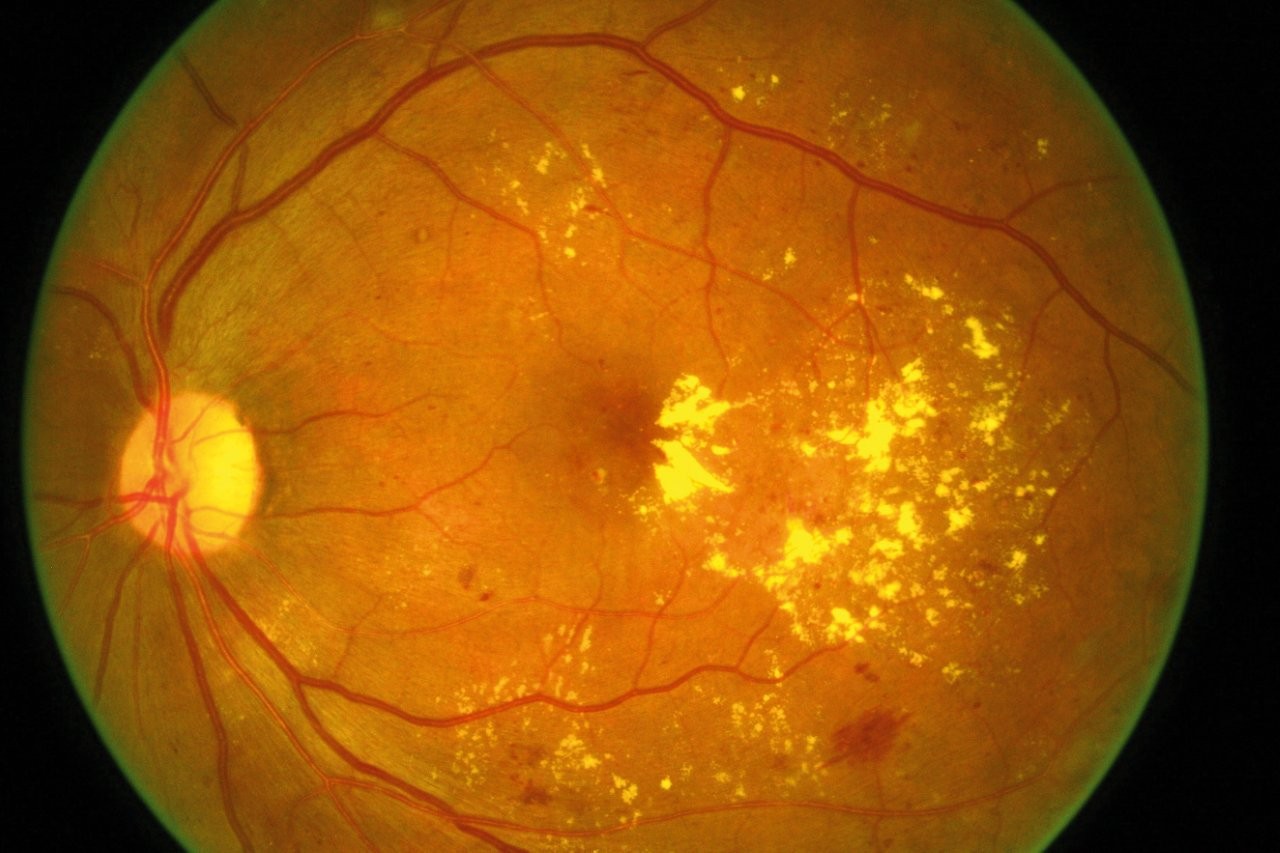
UK company Exonate announced plans to initiate a phase 2b clinical trial to further evaluate the clinical efficacy, optimal dosing and safety profile of EXN407, a twice-daily eye drop, in patients with non-proliferative diabetic retinopathy (NPDR).
The Clear-DE (clinical evaluation of a new eye drop for alleviating retinopathy in diabetic eye disease) trial will commence in early 2026, with 140 patients currently enrolled across sites in Australia, the Middle East and China.
In March 2024, the phase 1a/2b trial of EXN407-treated patients showed highlighted signals of biological activity (macular thickness reduction and decrease in retinal vascular leakage) and only mild adverse events, none of which led to treatment discontinuation, the company said.
EXN407 is a selective SRPK1 inhibitor with the potential to become the first effective eye-drop therapy for NPDR and diabetic macular oedema (DMO). “This therapy could transform the treatment landscape for early-stage disease by providing clinical benefit while avoiding the burden of injections, representing a significant advancement for patients and physicians alike,” said Dr Catherine Beech, Exonate CEO.