Finally, an affordable end to trichiasis
After more than three years of development and five years wading through regulatory paperwork, a new device to permanently remove ingrown eyelashes (trichiasis and districhiasis) is now available to buy in New Zealand and Australia.
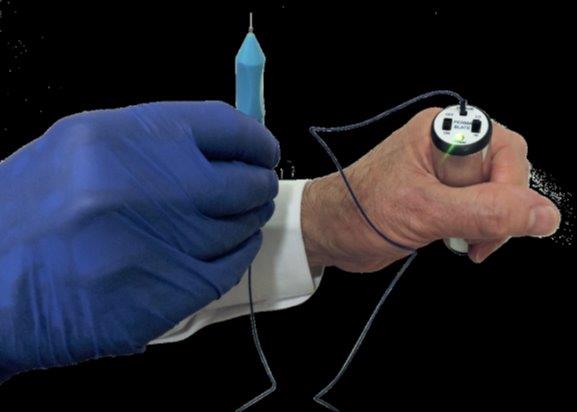
Permablate is a precise, portable and affordable electrolysis device which permanently ablates the germinal follicles of ingrowing eyelashes and eyelid margin metaplastic hairs, said Ignatios Koukouras, national product manager of distributor Designs for Vision (DFV). “Traditional larger-scale electrolysis devices can cause collateral tissue damage and necrosis. Permablate offers an affordable, safer treatment, greatly reducing the risks.”

DFV's Gian Victoria and Ignatios Koukouras proudly displaying the newly available Permablate electrolysis system at RANZCO 2022
Permablate was developed by the late trans-Tasman eye health educator, entrepreneur and DFV founder Richard Grills, former Sydney Eye Hospital head of oculoplastic surgery Associate Professor Ross Benger and Australian GP-turned-inventor Dr Stuart Esnouf. Other treatment methods are not localised sufficiently, leading to the loss of normal hairs; are often ineffective, regardless of treatment length; overly destructive of perifollicular eyelid tissue, due to the intensity and speed of treatment; and are very expensive, said A/Prof Benger, whose frustration with these issues led to Permablate’s development.
 A/Prof Ross Benger, who co-developed Permablate with Richard Grills and Sr Stuart Esnouf
A/Prof Ross Benger, who co-developed Permablate with Richard Grills and Sr Stuart Esnouf
Traditional electrolysis systems can cost several thousand dollars, compared with several hundred for Permablate, said Koukouras. “Permablate uses a fine, gold-plated 100µm needle and two power levels to necrotise the germinal follicle and comes with five disposable probes and a rechargeable lithium battery.”
In 2017, after completing an initial trial with 50 patients, and before realising how long the regulatory hurdles would take (aggravated by the Covid pandemic), an excited Grills summed up Permablate as being "economical, practical and non-invasive, which is what every good device should be. It’s a terrific little product and it’s really going to meet a niche.”
A/Prof Benger said he’s very pleased Permablate has finally been approved for medical use and sale in Australia, New Zealand and the US, but is sad the approval came after Grills’ death in July 2022.