Luxturna funding application stalls
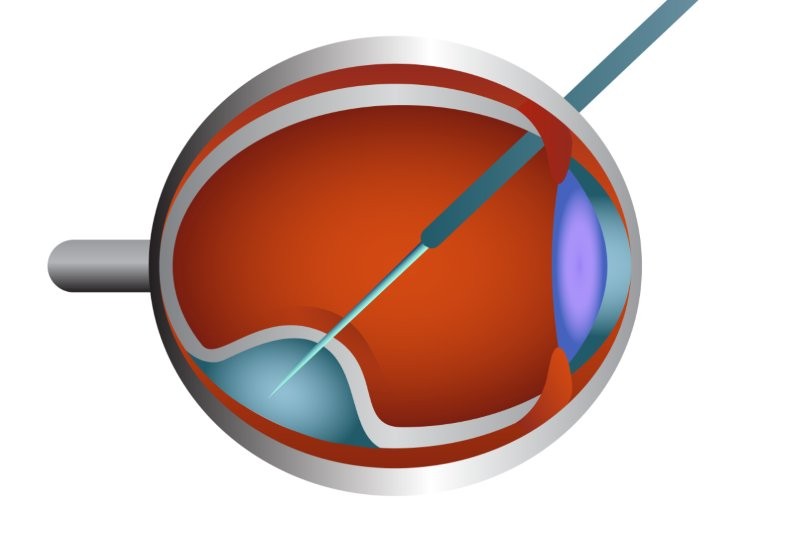
Pharmac’s deferment of its decision to fund Luxturna (voretigene neparvovec), a gene therapy to treat RPE65 mutation-associated retinal dystrophy, is “disappointing”, said the University of Auckland’s Associate Professor Andrea Vincent.
In July 2023 Pharmac announced, “The Committee recommended that voretigene neparvovec be deferred until further evidence regarding the longevity of the therapeutic benefits becomes available.” This is despite the minutes of its February 2023 meeting citing papers containing 7.5 years of longitudinal data about Luxturna, said A/Prof Vincent, who highlighted these data in a written response to the body in October.
Pharmac’s minutes also cited concerns that the drug prevents further vision loss but does not improve vision and noted the follow-up to Russell et al’s 2017 study showed Luxturna-treated patients’ visual acuity improvements were maintained for one to three years but reverted to pre-treatment levels by year four. “The committee noted that the standard of evidence required to support assumption of treatment effects beyond three years’ duration in humans was not yet available.”
Pharmac’s committee said it considered the health benefits reported in the trials are likely to be beneficial for family and whānau in addition to the individual receiving treatment but noted that voretigene neparvovec must be prepared in a pharmaceutical compounding setting capable of handling and preparing adeno-associated viral vector-based gene therapy products – a facility which may not yet be available in New Zealand. “The Committee considered that significant upskilling and training of involved personnel would be required to implement this service,” and noted just three eligible New Zealanders would benefit from Luxturna treatment at present. Although the meeting’s minutes stated the most likely location for such a facility would be in Auckland, where there is an existing IRD (inherited retinal disease) specialist clinic, “the Committee considered that this may be an additional barrier for people who live rurally, or who must take extended trips to visit the centre where the treatment is available.”
Caroline De Luca, Pharmac’s acting director of advice and assessment, said Luxturna is the first gene therapy to be considered by the committee. The challenges associated with appraising the value of such gene therapies are due to the high pharmaceutical costs, the uncertainty in the duration of treatment effect, the health-related quality of life benefits and the potential need for re-treatment if the treatment effect wanes, she said. “When making funding decisions we have to prioritise what treatments to fund. This process is dynamic as we consider health need, health benefit, cost/savings and suitability. The priority of one proposal compared to another can change as we receive further evidence and as we negotiate with suppliers. Because of this we’re unable to give a definitive timeframe for if, or when, funding decisions will be made.”
Luxturna has been funded in Australia since 2022, with a recent report estimating IRDs cost the country between AU$781m and AU$1.56bn every year.