Ozurdex ‘real-life’ results - effective for DMO
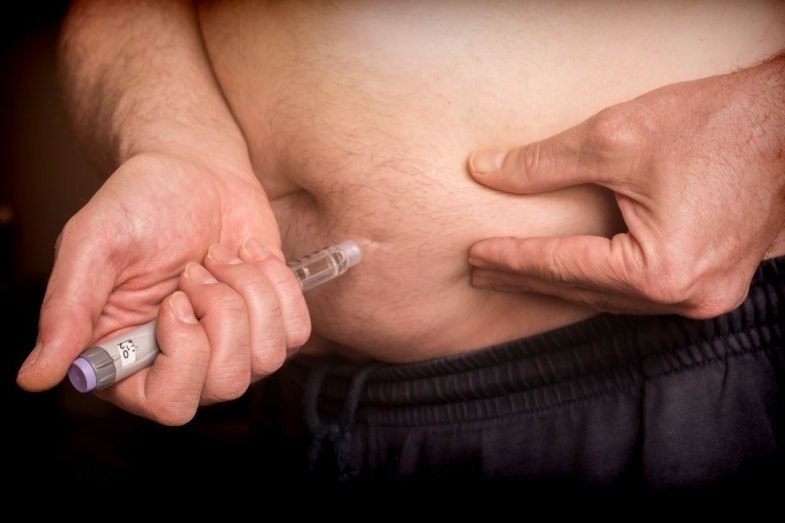
Aussiedex phase IV results trialling Ozurdex (dexamethasone) intravitreal implant in patients with diabetic macular oedema (DMO) echo existing evidence that Ozurdex is a safe and effective treatment for DMO.
The results showed improved outcomes across two patient populations; in the first-line setting, as well as those refractory to anti-VEGF (vascular endothelial growth factor) treatment.
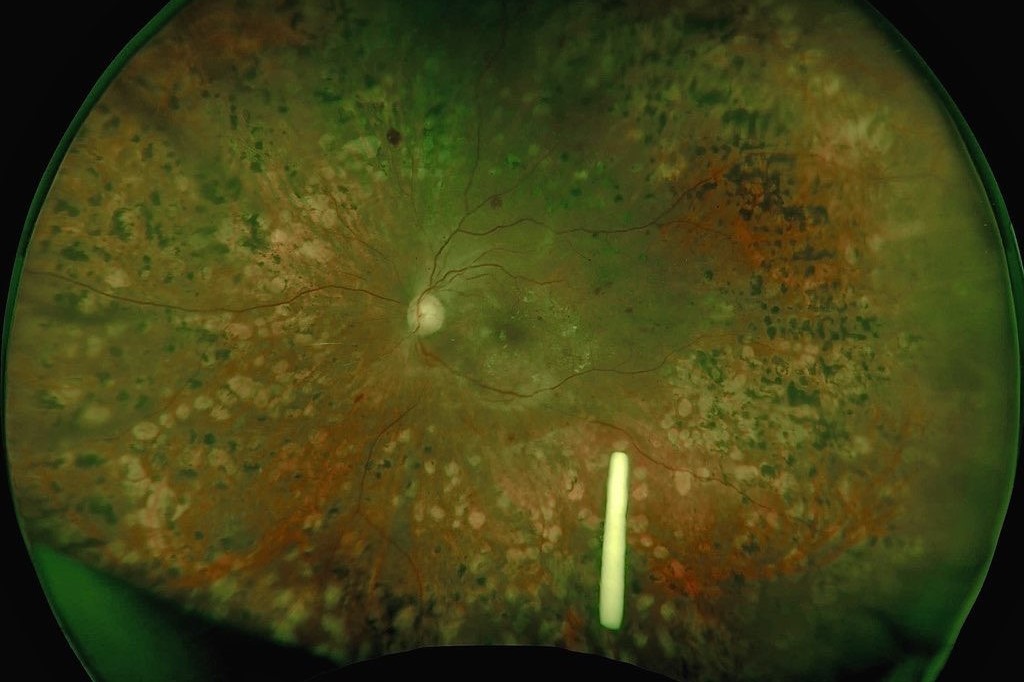
The open-label, prospective study, including 200 patients with vision loss due to DMO, found that Ozurdex significantly improved both best-corrected visual acuity (BCVA) and central macular thickness (CMT) at 12 months for the 55 treatment-naïve patients (p=0.042 and p<0.001, respectively). For this group, the Ozurdex treatment achieved both its primary endpoints and significant improvements were demonstrated as early as week six for both BCVA and CMT.
For the 142 patients refractory to anti-VEGF treatment, Ozurdex significantly improved CMT at 12 months (p<0.001), while the mean change in BCVA was significantly improved at week six and 24 (p<0.001).
Ozurdex’s safety and tolerability profile was consistent with previous clinical trials. Adverse reactions included increased intraocular pressure (IOP) that required treatment in 25% of treatment-naïve patients at 12 months, leading to one treatment discontinuation and no serious ocular adverse events. In the treatment-refractory group, increased IOP required treatment in 20% of patients at 12 months, leading to one treatment discontinuation. One increase in IOP was deemed a serious treatment-related adverse event and was resolved with medication.
Associate Professor Samantha Fraser-Bell, University of Sydney, presented the findings at the EURETINA 2019 congress in Paris. “Today’s results are significant for clinicians and patients alike, furthering our understanding of Ozurdex as a safe and effective treatment for DMO,” she said.