Pathological myopia
Pathological myopia is the presence of structural changes in high myopia, driven by axial elongation and posterior staphyloma1. High myopia is defined as a highly negative refractive error (> -6 to -8 dioptres), along with axial elongation (>26–26.5mm), while posterior staphyloma is defined in Spaide et al’s Pathologic Myopia (2021) as “an outpouching of the wall of the eye that has a radius of curvature that is less than the surrounding curvature of the wall of the eye”. This is the most common finding of pathological myopia, although occasionally posterior staphyloma can be present in eyes with normal axial lengths.
In pathological myopia, myopic maculopathy is an important cause of vison loss accounting for 12.2% of vision loss in Japan and is the fourth leading cause of vision loss in Spain (after age-related macular degeneration, glaucoma and diabetic retinopathy)1. Here, I present eight key points to aide your clinical practice in pathological myopia. Here, I present eight key points to aide your clinical practice in pathological myopia.
Recognise the key findings of a myopic fundus
The diagnosis of pathological myopia can be missed in patients with previous laser refractive surgery or cataract surgery and previous high myopia, as their refraction may be close to emmetropia. Clinicians must recognise the classic signs of a myopic fundus on examination, Optos ultra-widefield imaging and optical coherence tomography (OCT) imaging to make the correct diagnosis. These are:
- Optic nerve is often tilted, small with parapapillary atrophy. OCT imaging can show incorrect segmentation
- Peripheral retina can appear thin and tessellated. This is best appreciated on Optos ultra-widefield imaging
- Macula can show posterior bowing on OCT, along with thinning of the choroid and sometimes tractional changes.
If these signs are present, one should find out if there is a history of high myopia and consider obtaining axial length measurements to confirm the diagnosis.
Grading of myopic maculopathy
Myopic maculopathy is graded as per Table 1, using the ATN (atrophy, traction and neovascularisation) system1.

Table 1. Grading of myopic maculopathy
In clinical practice, this grading system is useful for clinicians to anticipate how the disease can progress and help guide the assessment so important signs are not missed. For example, clinicians need to be vigilant to the presence of active neovascularisation if there are lacquer cracks and new onset of distortion, or be mindful of the development of a full-thickness macular hole when there is foveal detachment.
How is myopic neovascularisation presenting?
Myopic neovascularisation can present without subretinal or intraretinal fluid (see Fig 1). These type II (subretinal) lesions present as an area of thickening in the outer retina with or without minimal subretinal fluid. With high-resolution OCT imaging, active lesions will have an area of ‘fuzziness’ around the border and the external limiting membrane can be interrupted. However, these subtle signs can be difficult to detect with lower resolution OCT systems. As a result, myopic neovascularisation can be difficult to diagnose. However, there are two tips to help:
- Myopic patients are very sensitive to distortion caused by neovascularisation, so the clinician should have a high degree of suspicion if the patient is symptomatic
- Comparison of current OCT imaging with previous scans often helps illustrate subtle neovascular changes.
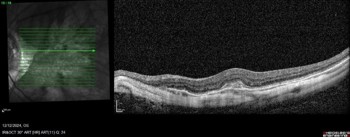
Fig 1. An example of a myopic neovascular membrane on OCT.
Notice that there is no associated subretinal or intraretinal fluid
Recognise signs of dome-shaped maculopathy
Dome-shaped maculopathy can cause subretinal fluid in pathological myopia without neovascularisation. It is caused by a bulge in the macular retina, choroid and retinal pigment epithelium (RPE) within the posterior staphyloma1. This is often associated with a localised shallow retinal detachment with subretinal fluid. The subretinal fluid responds poorly to anti-VEGF therapy and photodynamic therapy2, but the fluid is indolent and visual acuity can remain stable for a long period of time. The diagnosis can be relatively easy to make if there is a clear dome detectable on OCT. However, the dome can be more ridge-like in some cases and, if that ridge is parallel to the OCT B-scan, the diagnosis can be missed unless both horizontal and vertical scans are performed.
How to treat myopic neovascularisation
Patients with myopic neovascularisation respond quickly to anti-VEGF injections, and often only one or two injections are required to achieve quiescence. In the Myrror study3, patients with myopic neovascularisation were randomised to aflibercept or sham treatment with a pro re nata approach. Those patients with myopic maculopathy did very well with aflibercept, requiring a median number of two injections within the first eight weeks and no further injections in the rest of the first year. As a result, ongoing maintenance injections are not routinely required for patients with myopic maculopathy, unless the patient is elderly with co-existing age-related macular degeneration.
Watch for punctate inner choroidopathy or multifocal choroiditis mimics
In a paper published in 20224, 11% of patients with patchy atrophy had unrecognised previous punctate inner choroidopathy (PIC) or multifocal choroiditis (MFC). PIC/MFC lesions are more common in those with macular neovascularisation (81.8% vs 33.9%) (see Fig 3). Signs signifying PIC/MFC included an elevation of the RPE by homogenous and medium hyperreflectivity material, often associated with disruption of the photoreceptor ellipsoid zone and interdigitation zone and/or choroidal hypertransmission below the lesions, along with mid-peripheral curvilinear scars and hyper-autofluorescence changes. It is important to recognise PIC/MFC early, as treatment with prednisone and systemic immunosuppression can slow or stop the disease process and prevent further vision loss (see Fig 2).


Fig 2. (L) Optos imaging of the right eye in a patient with pathological myopia and PIC. Note the atrophic lesions which resemble patchy atrophy in myopia and lacquer cracks radiating to the macula
Fig 3. (R) A fluorescein angiogram showing the left eye of a patient with pathological myopia, posterior staphyloma and myopic maculopathy. The retinal vessels terminated at the edge of the posterior staphyloma with significant amount of peripheral non-perfusion but no neovascularisation
Pathological myopia associated with significant peripheral vascular changes
As Optos imaging becomes more widely available, these mostly benign peripheral retinal findings in patients with pathological myopia are being detected more frequently. In a paper published in 20145, peripheral capillary telangiectasia was a common finding in both emmetropic and myopic eyes (around 80%). However, peripheral retinal non-perfusion was present in 82.6% of myopic eyes vs only 4.8% of emmetropic eyes. Furthermore, the perfused area was limited to just beyond the staphyloma border and none of these eyes developed neovascularisation (see Fig 3).
Be aware of optic nerve pathology in myopic patients
High myopia is a risk factor for glaucoma, and it is associated with longer axial length, larger optic disc size and/or larger parapapillary delta zone. Furthermore, a non-glaucomatous optic neuropathy can develop from parapapillary gamma-zone lengthening of the retinal nerve fibre affecting the papillomacular region.
However, assessment of optic neuropathy in pathological myopia is challenging. Structural assessment of the parapapillary retinal nerve fibre layer with OCT is unreliable due to segmentation error. Some authors advocate assessment of the retinal nerve fibre layer and ganglion cell layer thickness on the macular OCT scan in these cases6, but if there is a staphyloma or coexisting myopic maculopathy the disturbance in the anatomy renders this assessment method unreliable. Pattern-reversal visual evoked potential (p-VEP) is traditionally used for the measurement of optic nerve function but, as this response is driven by macular function, the reliability of this measurement is, again, limited if there is myopic maculopathy. The photopic negative response on the full-field electroretinogram has been used to assess optic nerve function, but this is a small amplitude response and intervisit variability can limit its usefulness.
Contact
Please do not hesitate to reach out to me by email lsheck@retinaspecialists.co.nz should you have any feedback or want me to cast an eye over a scan in these often challenging cases.
References
- Jorge Ruiz-Medrano, Javier A. Montero, Ignacio Flores-Moreno, Luis Arias, Alfredo García-Layana, and José M. Ruiz-Moreno. Myopic maculopathy: Current status and proposal for a new classification and grading system (ATN). Progress in Retinal and Eye Research, 69:80–115, March 2019.
- Mukesh Jain, Lingam Gopal, and Tapas Ranjan Padhi. Dome-shaped maculopathy: a review. Eye (Lond), 35(9):2458–2467, Sep 2021.
- Yasushi Ikuno, Kyoko Ohno-Matsui, Tien Yin Wong, Jean-Francois Korobelnik, Robert Vitti, Tummy Li, Brigitte Stemper, Friedrich Asmus, Oliver Zeitz, and Tatsuro Ishibashi. Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: The Myrror study. Ophthalmology, 122(6):1220–1227, Jun 2015.
- Shymaa K. Hady, Shiqi Xie, K. Bailey Freund, Emmett T. Cunningham, Chee Wai Wong, Chui Ming Gemmy Cheung, Koju Kamoi, Tae Igarashi-Yokoi, Omar M. Ali, Ehab I. Wasfi, Mahmoud F. Rateb, and Kyoko Ohno-Matsui. Prevalence and characteristics of multifocal choroiditis/punctate inner choroidopathy in pathologic myopia eyes with patchy atrophy. Retina, 42(4):669–678, April 2022.
- Yuichiro Kaneko, Muka Moriyama, Shuichiro Hirahara, Yuichiro Ogura, and Kyoko Ohno-Matsui. Areas of nonperfusion in peripheral retina of eyes with pathologic myopia detected by ultra-widefield fluorescein angiography. IOVS, 55(3):1432, March 2014.
- Yoon Jeong, Young Kook Kim, Jin Wook Jeoung, and Ki Ho Park. Comparison of optical coherence tomography structural parameters for diagnosis of glaucoma in high myopia. JAMA Ophthalmol, 141(7):631–639, Jul 2023.

Dr Leo Sheck specialises in medical retina, genetic eye disease, electrodiagnostics for complex retina and optic nerve diseases and cataract surgery, especially with co-existing retinal diseases. He consults for Te Whatu Ora Auckland and Retina Specialists and is a director of the NZ Save Sight Society.