Synthetic scleral patch approved
The world’s first synthetic and non-degradable ophthalmic tissue-integrating substitute, EverPatch, should be available in the US by the end of 2023, said its manufacturer CorNeat Vision.
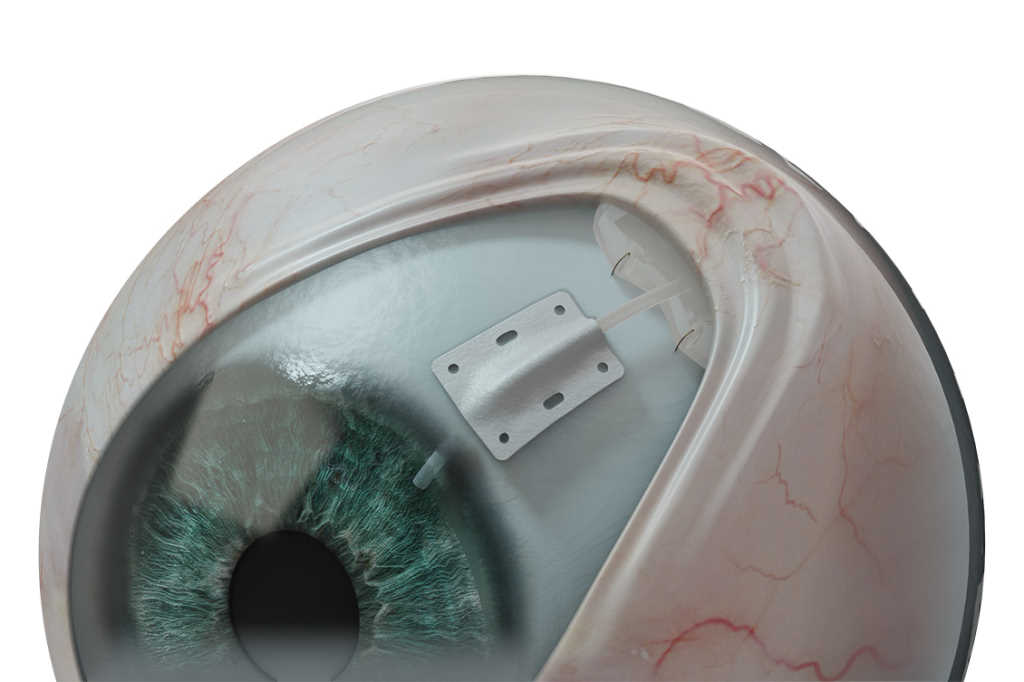
Composed of a non-woven, polymer matrix which integrates with surrounding tissue, EverPatch is intended to reinforce the sclera and aid the physical reconstruction of the ocular surface. CorNeat Vision co-founder and chief medical officer Dr Gilad Litvin said the patch is significantly thinner than processed patch tissue and provides better handling, as it does not ‘cheese wire’ when sutured, and has holes that allow for accurate positioning and anchoring.
Everpatch has been granted US FDA 510(k) clearance, while an ongoing trial is geared towards European CE approval for wider indications, including the concealment of glaucoma shunts and scleral sutures used in fitting IOLs, by early 2024, said CorNeat.