Trial examines presbyopia drop Vuity
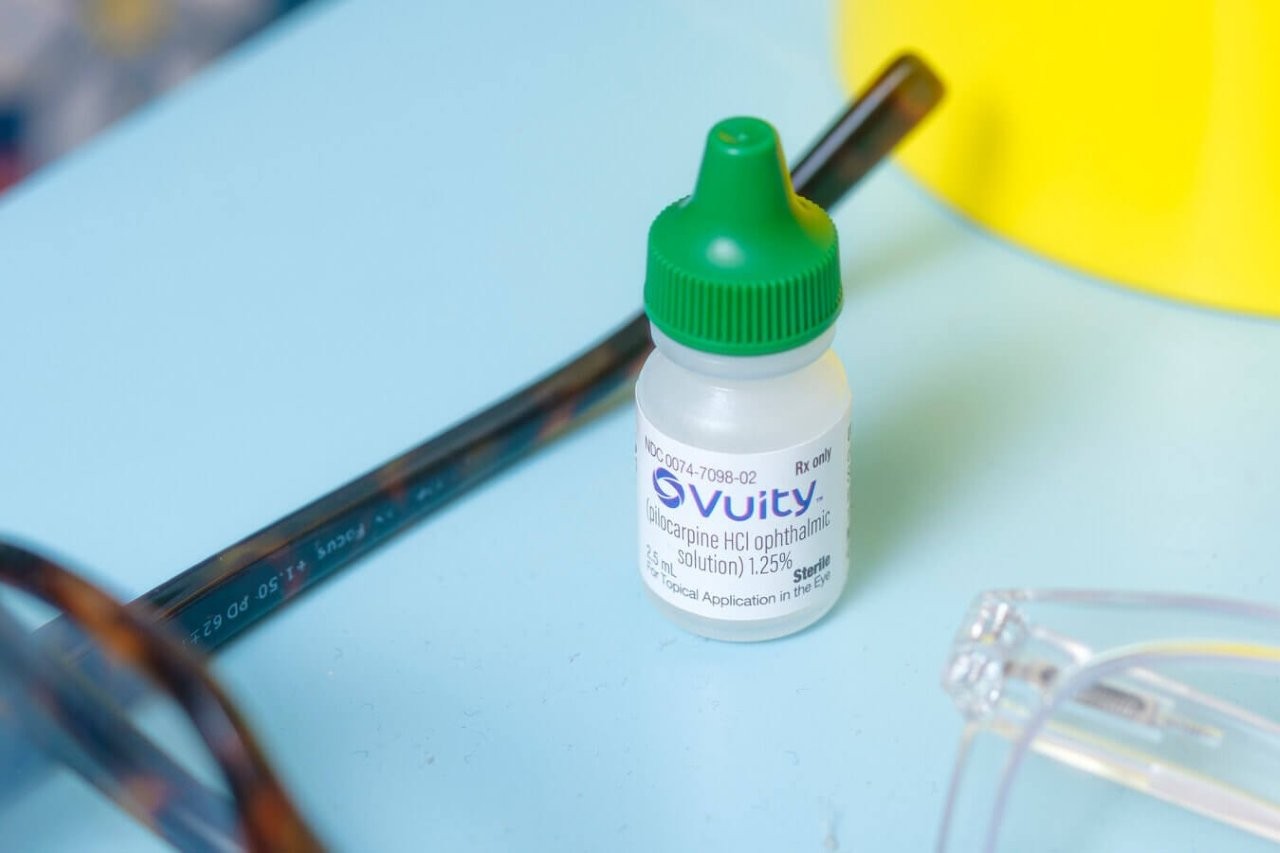
Building on previous work researching Vuity eye drops’ (pilocarpine 1.25%) efficacy and mode of action for presbyopia, University of Auckland researcher Dr Alyssa Lie is poised to start a new trial examining pilocarpine’s broader pharmacological effects on the eye.
Working with co-investigators Drs Lucy Goodman and John Phillips from the School of Optometry and Vision Science (SOVS), Dr Lie said the study is the first to examine Vuity’s longer-term use. “The Gemini 1 and 2 clinical trials, which preceded the FDA’s approval of Vuity, demonstrated a favourable safety profile with up to twice-daily use of the eye drops over a one-month period… our study is the first (to our knowledge) to examine its use over a longer duration, specifically, a three-month period, surpassing the length of the Gemini trials.”
The trial will use electrophysiology and optical coherence tomography (OCT) to examine any changes to retinal structure or function over the three months. Patients using the eye drops will be compared against control patients who use spectacles for presbyopia. “Vuity eye drops treat presbyopia by dynamic pupil modulation,” explained Dr Lie. “This increases depth-of-focus to reduce the amount of perceived blur. However, it does not optically correct any defocused light (as spectacles do). Preliminary evidence suggests that optical defocus can alter choroidal thickness by affecting choroidal blood flow, which may, in turn, influence retinal function.”

Dr Alyssa Lie
Other studies show that atropine, an antagonist of pilocarpine, also affects choroidal thickness by modifying blood flow within the choroid, she said. “Understanding these changes is crucial, as they could provide insight into potential long-term effects of Vuity treatment and help assess the broader implications for eye health and retinal function over time.”
SOVS optometrist Roberta Gough will help conduct the mechanistic aspect of the study as part of her master’s degree, said Dr Lie, who is currently recruiting for the trial. “We are looking for healthy individuals aged between 40 and 55 experiencing presbyopia but otherwise have good unaided distance vision and eye health.”
The trial is being funded by an Association of Research in Vision and Ophthalmology Foundation Catalyst Award as well as a “generous” charitable donation from the Freemasons Foundation, she said. “As clinicians, we should embrace new treatments, but it's equally important to approach their use with caution. Our study aims to contribute to this careful, evidence-based approach, ensuring our enthusiasm for progress is balanced with a rigorous evaluation of the data.”