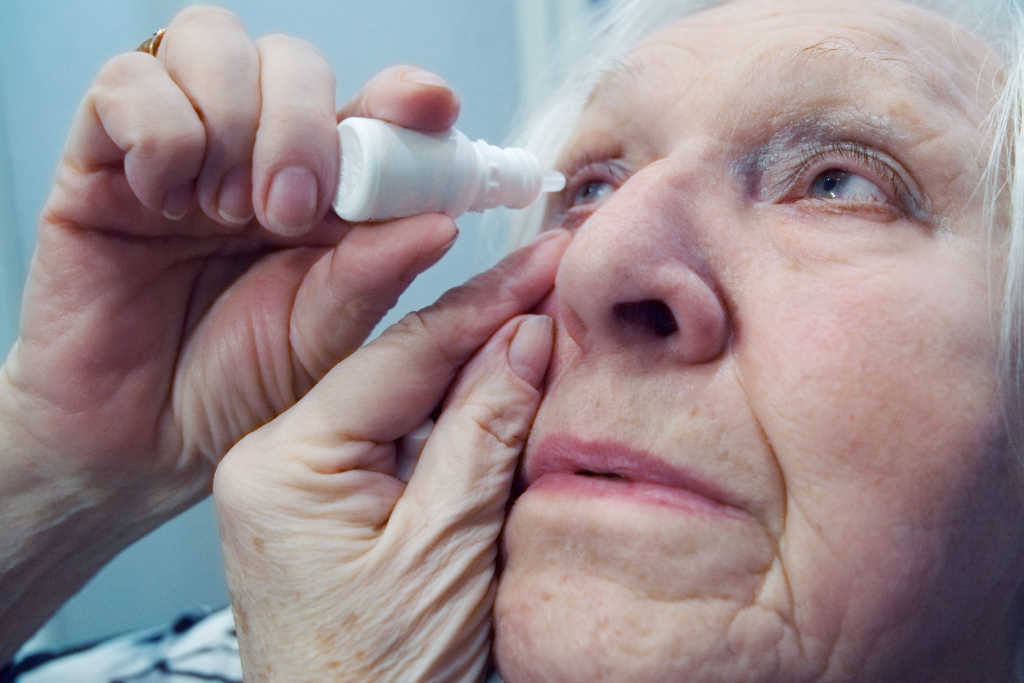
Unravelling NZ’s lack of preservative-free drops
At the 2021 glaucoma-focused Ocular Therapeutics Evening (OTE), Auckland-based ophthalmologists Drs Hussain Patel and Divya Perumal highlighted New Zealand’s dearth of preservative-free eye drop options for their glaucoma patients.
According to Eye Institute’s Dr Perumal, preservatives can cause dry eye, blepharitis, keratitis, uveitis and conjunctival scarring. In glaucoma patients this can adversely affect patient compliance and the success of any future surgery (eg. trabeculectomy); as such, they increase the risk of blindness. Dr Perumal advised colleagues to try alternatives with fewer preservatives, such as Travopt and Travatan, both containing the prostaglandin travoprost and funded by the government’s Pharmaceutical Management Agency (Pharmac).
“We are most in need of a range of preservative-free glaucoma eye drops, since a significant proportion of our patients get intolerance or side-effects to the preservatives,” agreed Auckland Eye’s Dr Shenton Chew. The problem, even with preservative-free drops with the same active ingredient, is that different brands use different carrier solutions, which may disagree with patients. There’s such limited choice in New Zealand that these alternatives aren’t available or funded here, he said, “So it would be nice to have the option of a range of branded-preserved drops too.”
The lack of access to alternatives has forced doctors and their Kiwi patients to source and pay for preservative-free eye drops from overseas – a workaround to which the pandemic has thrown up barriers, said Eye Surgery Associates’ Dr Patel.

Drs Divya Perumal, Graham Reeves and Hussain Patel
Although New Zealand ophthalmologists can access the preservative-free versions of timolol, bimatoprost (Lumigan), bimatoprost + timolol (Ganfort) and Minims pilocarpine nitrate 2%, only the latter is Pharmac-funded. Unfortunately, pilocarpine also has many side effects, said Dr Perumal, including irritation of the eye, headaches, miosis and retinal detachment – so the only funded preservative-free glaucoma drop available in New Zealand has limited use.
Two particular examples highlighted as missing from Kiwi ophthalmologists’ armamentarium at the OTE were loteprednol, a corticosteroid to treat eye inflammation, and tafluprost, for the treatment of open-angle glaucoma and management of ocular hypertension. Tafluprost (Zioptan, Merck) was Food and Drug Administration (FDA) approved for use in the USA in June 2021. Loteprednol would be particularly useful in uveitis patients, for example, said Dr Perumal, because compared with most steroids it has a lower risk of increasing intraocular pressure (IOP) and potentially causing glaucoma.
Preservative-free versions of most glaucoma drops are available overseas, so why not in New Zealand?
The chicken or the egg?
Courtenay Kularatne, a product regulation advisor at Medsafe, wouldn’t comment on why this is the case, only confirming that currently there were no medicines in New Zealand that contain either loteprednol or tafluprost. But for a drug company trying to sell its product in New Zealand, applying for Medsafe approval is just the beginning.
In terms of loteprednol, Pharmac may have been waiting for a New Zealand distributor to step up and make it available so an application can be put forward, suggested Eye Institute’s Dr Graham Reeves. “It’s a bit chicken and egg, as companies are unlikely to want to distribute something with limited market if it’s unsubsidised!”
Conor Fitzgerald, brand manager for Radiant Health, an Auckland-based partner of Bausch+Lomb, which produces Lotemax (loteprednol), said, “We tested the market for demand of Lotemax in both Australia and New Zealand, but it was not large enough to warrant registration of the product in either country. We do, however, supply Lotemax in New Zealand under section 29 of the Medicines Act (supply of unapproved medicines).”
Pharmac’s role
In Budget 2021, Pharmac’s $1.1 billion budget was increased by $200m, spread over the next four years. That is spent on funding medicines for 3.73 million New Zealanders every year, according to Pharmac. While the agency typically receives funding applications from pharmaceutical companies, doctors and patients can also apply for a Medsafe-approved drug to be funded.
Lisa Williams, Pharmac’s director of operations, said the agency hadn’t had an application as yet for tafluprost but it had had one for loteprednol in 2014. “But as there was no registered product of loteprednol in New Zealand we were unable to progress the application. We would be able to revisit it if a registered product became available.”
On Pharmac’s drugs list, loteprednol’s application is currently listed as “seeking clinical advice”. In September 2017, Pharmac’s Pharmacology and Therapeutics Advisory Committee’s (PTAC’s) ophthalmology subcommittee noted that, “Lotemax (loteprednol etabonate 0.5%) is available to patients via the private market. The subcommittee noted that at the time of this discussion, this product was neither registered nor pending registration with Medsafe in New Zealand.” The subcommittee then recommended that Pharmac staff seek suppliers of loteprednol eye drops to enter the New Zealand market and that loteprednol be listed with high priority.
“The process is quite frustrating,” said Dr Patel. “We did try quite hard to get loteprednol into New Zealand but gave up eventually. It is interesting that no one has put an application in for tafluprost. I guess that would have to come from the manufacturer (Merck) but it wouldn’t be worth doing if there is no chance of success.”
Dr Reeves said he had assumed funding applications for preservative-free drops such as these, and many of the fixed-combination formulations such as bimatoprost and timolol, travoprost and timolol, had simply been rejected. “I guess people then stop trying if it seems futile, hence a paucity of recent applications. Most of us here would love the range of fixed combinations available overseas to give us more options and improve our patients’ adherence and quality of life by often only needing once-daily treatment.”
Interestingly, however, there was recent approval of Arrow-Lattim (a latanoprost and timolol combination to reduce intraocular pressure in patients with open-angle glaucoma), said Dr Reeves. “It came onto the subsidised formulary with little to no fanfare, so many doctors are unaware it is now available.” According to Medsafe, however, Arrow-Lattim contains the preservative benzalkonium chloride.