Amniotic membrane for dry eye?
A study published in Clinical Ophthalmology suggests a single application of self-retained cryopreserved amniotic membrane (CAM) can speed corneal surface recovery and help reduce symptoms of dry eye disease (DED) for up to three months.
A retrospective study conducted at 10 clinical sites in the United States, comprising 84 patients (97 eyes) whose symptoms were severe (SCALE) despite maximal medical treatments with artificial tears, steroids, cyclosporine, antibiotics, serum drops and nonsteroidal anti-inflammatory drugs (NSAIDS).
CAM was applied for between two and 11 days (average five) and was well tolerated in all but four eyes. It was removed from these after two days. 88% of patients had a reduction in symptoms and significant improvement of the ocular surface after CAM removal.
The overall Dry Eye Workshop (DEWS) ocular discomfort severity score was significantly reduced from 3.25±0.5 at the baseline to 1.44±0.6 at 1 week (p<0.001), 1.45±0.6 at 1 month (p<0.001), and 1.47±0.6 at 3 months (p<0.001). Specifically, ocular discomfort scores improved from 3.0±0.8 at baseline to 1.3±0.7 at 3 months.
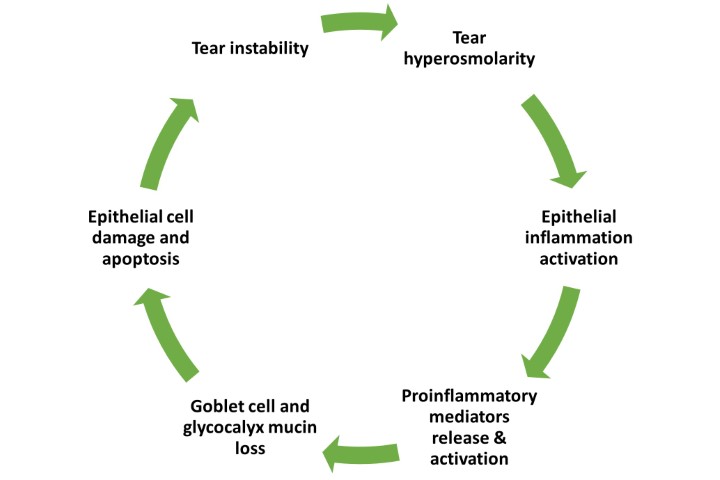
The study, led by Drs Marguerite McDonald and Hosam Sheha, who are consultants for BioTech, makers of the Prokera Slim CAM used in the study, points out inflammation has been recognised as a key component of dry eye disease, and CAM is known to have potent anti-inflammatory effects.
They say the therapeutic effect of CAM in the treatment of DED can be attributed to multiple mechanisms of action, which collectively seem to be beneficial in treating DED.
Firstly, CAM acts as a therapeutic bandage that keeps the eye moist by retaining tears and protects the ocular surface from the surrounding environment; secondly, it controls ocular surface inflammation; and thirdly, CAM’s ability to regenerate corneal nerves may explain the lasting effect.
Nerve Growth Factor (NGF) is abundantly present in CAM and is known to play an important role in nerve regeneration and epithelial healing, say the authors, while other conventional topical anti-inflammatory therapies such as cyclosporine, corticosteroids, or NSAIDs have been found to compromise corneal nerves and may explain why some cases do not respond.
“The clinical evidence presented in the DRy Eye Amniotic Membrane (DREAM) study continues to further establish the effectiveness of cryopreserved amniotic membrane in supporting healing in patients, and our commitment to provide tissue products that address the entire ocular surface disease spectrum,” said Adrian Roji, chief commercial officer of BioTech's parent company, TissueTech. “Our cryopreserved amniotic tissue supports the healing of the ocular surface, and is also promising with respect to corneal nerve integrity.”
Further studies are needed, say the authors, to determine longer-term effects (>3 months) and whether repetitive use of CAM generates a more lasting effect.
Interested in Dry Eye? NZ Optics’ annual Dry Eye Special will be available on the this website from 16 September 2018.