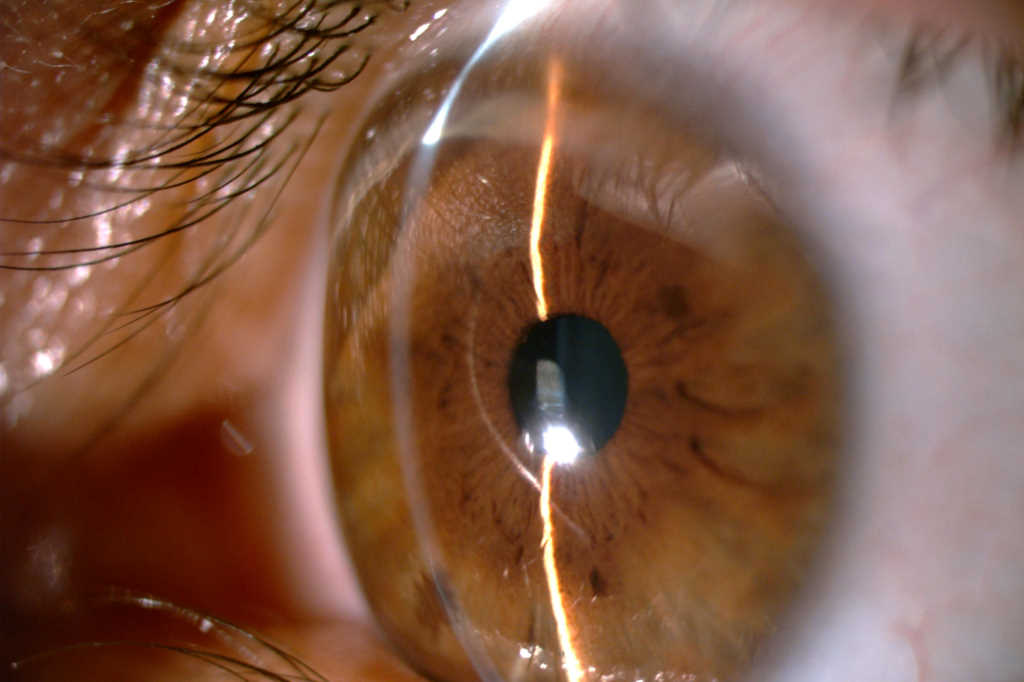
Current state of keratoconus management in New Zealand
Keratoconus is a progressive corneal ectasia, characterised by corneal thinning and protrusion, in a cone like manner, causing irregular astigmatism1. The reported prevalence of keratoconus varies between ethnicities with high rates in Middle Eastern, Indian and Māori populations2. In New Zealand, a paediatric population-based study estimated that Māori ethnicity had prevalence rates four times higher than the general population (2,250/100,000 vs 520/100,000)3. Keratoconus is a complex clinical entity where late diagnosis, poor surveillance and delayed treatment can result in preventable vision loss, disproportionately impacting patients’ quality of life and adversely affecting public health resources2. Therefore, management of keratoconus includes but is not limited to diagnosis, referral, clinic attendance, treatment, access and cost, and is important to improving eye health outcomes and inequity in New Zealand.
Data from the New Zealand National Eye Bank over the past two decades have consistently reported keratoconus as the leading indication for corneal transplantation, accounting for 40-45% of corneal transplants annually (Fig 1)4. Over the last two decades, corneal crosslinking (CXL) has revolutionised the management of keratoconus by effectively stabilising the underlying ectatic process, with a reported success rate of 73.3-100%5. CXL is a technique used to strengthen corneal tissue (Fig 2). CXL utilises riboflavin (vitamin B2) as a photosensitiser and ultraviolet-A light (UVA) to ultimately slow or prevent disease progression in patients with keratoconus by creating covalent bonds within the corneal stroma, increasing stiffness, which prevents the progression of keratoconus5. Therefore, the earlier progressive keratoconus is referred to an ophthalmologist for CXL, the earlier disease progression may be halted, preventing moderate to severe vision loss.
Analysing diagnostics
Keratoconus is a challenging disease to characterise; however consensus has been reached that keratoconus can be defined by an abnormal posterior ectasia, abnormal corneal thickness distribution and clinical noninflammatory corneal thinning6. To diagnose keratoconus progression, an increase in anterior and posterior corneal curvature and corneal thinning greater than the noise of the corneal imaging device is required6. Corneal imaging devices include topographers, which analyse the shape of the anterior surface of the cornea, and tomographers which analyse both the anterior and posterior surfaces of the cornea and corneal thickness distribution. These imaging devices are not always accessible in primary care due to the expense of purchase7. In addition, keratoconus progression can be indicated by worsening visual acuity, change in spectacle refraction and the development of clinical signs such as Fleischer rings, Vogt's striae, acute corneal hydrops or apical scarring. However, early or mild progression is often present without the aforementioned signs and patients require regular topographic evaluation to monitor for progression.
A recent study conducted by our team identified that the three commonly used corneal imaging devices in keratoconus diagnosis and monitoring - the Medmont-E300, Revo-NX and Pentacam-AXL - do not have a high level of agreement but do have high intra-device repeatability8. This suggests that most patients when referred to a tertiary centre may require a new baseline assessment if imaged with a different device. They then may need to be observed for a period of time to ensure evidence of progression before further intervention, if not already shown on repeated scanning prior to referral. This adds a potential barrier to receiving treatment, as it could create several more appointments patients need to attend prior to receiving CXL. Furthermore, corneal imaging devices are not universally available in the community, with only 56% of optometrists owning such devices and, of these, 81% were topographers which do not provide data on corneal pachymetry or posterior corneal elevation9. In addition, New Zealand lacks standardised guidelines on the referral criteria for patients for tertiary assessment and consideration for CXL, creating ambiguity and potentially resulting in heterogeneity in care received by patients.
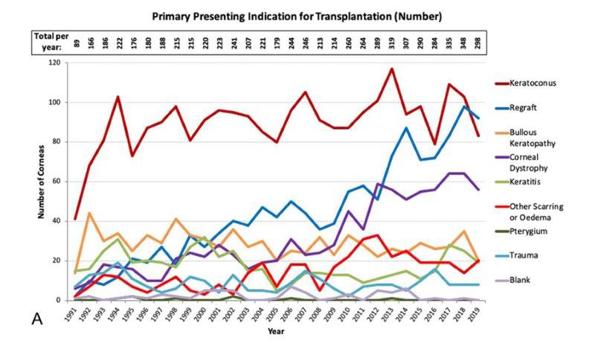
Fig 1. Primary presenting indication for corneal transplantation4
Preserving and supporting sight
In its mildest form, keratoconus presents as a mild astigmatism, correctable through spectacles and contact lenses. However, as keratoconus is a progressive disease, it may progress past this, whereby specialised contact lenses such as rigid gas permeable, scleral or hybrid lenses are required. Some scarring or protrusion of the cornea cannot be correctable by even a specialised contact lens; in these cases, a corneal transplant is considered.
A New Zealand study identified that management of keratoconus in the community varied among optometrists. The study identified that a combination of history and visual acuity, manual keratometry, slit lamp signs and corneal topography were reported as important in keratoconus diagnosis by 71% of optometrists9. However, 3.7% made a diagnosis on history and visual acuity alone, 5.6% with only manual keratometry and slit lamp signs and 19.9% with only corneal topography and slit lamp signs. This shows significant variability in the diagnostic criteria used in the community and perhaps further reflects limited access to corneal imaging units.
Keratoconus encompasses a complex disease process where progression typically occurs despite stable slit lamp signs and in the absence of reduced visual acuity10. In addition, isolated posterior surface corneal elevation can be the first sign of keratoconus progression10. Therefore, tomographic imaging would be the ideal modality to diagnose keratoconus and monitor for progression.
Referral patterns by optometrists were also highly variable, with 41.2% of optometrists referring on the progression of corneal parameters from topography, followed by 27.2% upon initial diagnosis, 21.2% at no set time and 9.9% on reduction of visual acuity. Most optometrists would refer for surgical opinion when vision was reduced to between 6/9 and 6/12. This study also highlighted that ethnicity was not a significant consideration in keratoconus referral – 57.3% of optometrists do not consider patient ethnicity when making a referral for CXL, despite the high prevalence and increased severity of the disease amongst Māori and Pacific peoples. However, 87.4% of optometrists did consider age at referral. Children are more likely to have aggressive disease and up to 20% will progress despite CXL11. Given the risk of progression in children with keratoconus, some have advocated treatment on diagnosis, even without documented progression11. The current referral patterns suggest a consensus among optometrists, with 87.4% considering age on referral; however, only 27.2% refer for CXL on initial diagnosis. To ensure adequate management of children with keratoconus, with the ultimate aim of reducing the number of patients with severe disease, we advocate that all patients under 18 years of age should ideally be referred upon initial diagnosis.
Referral is only the first step
Once patients were referred, a low attendance rate has been identified, especially among Māori and Pacific peoples (64% and 59%, respectively)12. Reduced attendance leads to worse disease severity due to resultant delay in undergoing CXL, requiring more expensive visual aids, Blind Low Vision NZ assistance and costs associated with complex surgical intervention. This highlights the importance of investigating the barriers to accessing care, which aims to target earlier referral to clinic, prompt treatment with CXL and less expensive visual aids and potential surgery12.
Possible barriers to accessing care were investigated and few were identified as correlating to poor attendance. These included factors relating to ethnicity, such as socioeconomic status and unemployment (correlating to worse visual acuity). Unsurprisingly, Māori and Pacific Peoples were found to present younger with worse disease severity and visual acuity. Although other factors have previously been identified as barriers to care (such as distance travelled to clinic, car ownership or family history), these were not significant in the population attending the Auckland keratoconus and crosslinking service.
Assessing the cost
The social consequences of non-attendance were marked with 16 subjects (14.5%), indicating that their disease impacted their employment (unpublished data). Furthermore, five subjects (4.5%) had to change their study courses due to keratoconus and 10 subjects (9.1%) said keratoconus was a hindrance to their employment, with most having to take a few days off work per annum (mean: 4.1 ± 6.4 (0-30) days, median: 2.0 (5) days). Chan et al reported a 30-fold increase in optical expenses, including costs for medical care, visual aids, private health insurance, productivity losses, transportation and other indirect costs, of patients with keratoconus compared to the general population13. This represents a significant unjust burden to this population.
Existing data have identified that Māori and Pacific peoples are disproportionately affected by keratoconus in New Zealand. Goh et al previously reported a mean wait time of 153 days for CXL treatment at Auckland District Health Board, with 40% showing signs of progression while on the waiting list2. This is in keeping with previous reports of a higher prevalence of keratoconus among Māori patients as well as a greater likelihood of requiring corneal transplant surgery at a significantly younger age compared with their European counterparts. Unpublished, updated data have identified there has not been substantial improvement over the last 10 years, with a new mean waiting period of 131 days but Māori are still the most likely to progress while waiting for CXL (48.5%).
Few studies have examined the economic impacts of keratoconus. Chan et al reported a yearly cost of AUD$3,365 per keratoconus patient and a total cost of approximately AU$44.7 million per year in Australia, using a patient health expenditure questionnaire13. Unpublished data has identified that in New Zealand the total direct and indirect costs averaged NZ$1,258 per keratoconus subject per year. The estimated lifetime per capita cost is NZ$79,254 and the total cost for keratoconus is estimated to be approximately NZ$30.9 million per year. Thus, compared to Australia the total out-of-pocket spend for keratoconus is less, despite New Zealand having a higher prevalence of keratoconus. This can be attributable to multiple reasons including a more socioeconomically deprived population, reduced access to services and a lack of support from government subsidies and low rates of private health insurance coverage.
The annual household income was reported to be less than AU$80,000 for 50% of the participants in Australia and less than NZ$70,000 for 50% of the population in New Zealand (approximately 20% less when accounting for currency conversion). Reduced income is associated with reduced cash flow, affecting direct and indirect costs associated with keratoconus. In the New Zealand study, 74.5% of subjects had never worn contact lenses, the mainstay visual rehabilitation management for keratoconus, and 32.7% had spectacles that were purchased over 24 months prior. This compares poorly to the Collaborative Longitudinal Evaluation of Keratoconus study in the US, where 74% of subjects wore contact lenses, and the Australian study, where 61% of subjects wore contact lenses daily7,14. This suggests either a lack of education on visual rehabilitation and available subsidies in New Zealand, a lack of financial resources to access contact lenses, or long waiting times in the public health system.
Cover by private health insurance in New Zealand was less than in Australia, with only 9% of subjects having private health insurance with optical benefits, compared to 55% in Australia. This may be due to a lack of support from insurance companies to fund optical benefits, or financial constraints of patients, as the most common reasons given for not having private health insurance were “too expensive” or perceived “poor value for money.” In addition, Australia has Medicare Benefits, a nationwide medical cover scheme which, for those enrolled, can be added to income tax covering some medical treatments, including optometrist fees, and offers rebates for private health insurance. In contrast, in New Zealand, there is little access to schemes covering the costs of an optometrist or GP visits and private health insurance is an out-of-pocket cost, whereas public hospital visits are free at the point of access.
In summary
Research has shown a lack of heterogeneity among community optometrists, with regard to the diagnosis and monitoring of keratoconus thereby affecting equity in delivery of care. Improved guidance and support for the optometric community is likely to reduce heterogeneity and improve equity. Furthermore, poor attendance is seen in tertiary care services disproportionately affecting our vulnerable populations, Māori and Pacific people. The cost of keratoconus is substantial and there is a lack of support from private health insurance and public health providers. This leaves our lower socioeconomic populations with worse disease severity and visual outcomes as they cannot afford private care, experience worse disease progression while on waiting lists due to an overburdened healthcare system with long waiting times and cannot afford visual rehabilitation.
These recent studies on the management of keratoconus in New Zealand highlight possible avenues for practical approaches for addressing eye health inequity and for future research. This includes support for community optometrists through national standardised guidelines which may consider age and ethnicity in referral, improved access to corneal imaging devices in the community and improved communication with ophthalmologists and tertiary centres regarding ongoing care and waitlist times. This could also be aided by more collaboration with societies such as RANZCO, NZAO and CCLS for specialty contact lens fitting workshops and incentivising and subsidising such workshops, and supporting our tertiary centres to improve access to clinics by expanding them to weekends or evening weekday clinics.
Advocating for increased funding from government organisations can directly target vulnerable groups through initiatives such as RANZCO Vision 2030 and Beyond, keratoconus screening and the University of Auckland’s Vision Bus Aotearoa, as well as targeting low-decile school screening programmes (currently in developmental stages) and increasing patient funding to access visual rehabilitation through optometrist visits and spectacle and contact lens subsidies. These initiatives could massively impact health inequities and would go a long way to making keratoconus a manageable disease, instead of a debilitating one, and improving eye health outcomes for our most vulnerable populations.
References
- Ziaei M, Barsam A, Shamie N, et al. Reshaping procedures for the surgical management of corneal ectasia. J Cataract Refract Surg 2015; 41:842-872.
- Goh YW, Gokul A, Yadegarfar ME, et al. Prospective clinical study of keratoconus progression in patients awaiting corneal cross-linking. Cornea 2020; 39:1256-1260.
- Papali'i-Curtin AT, Cox R, Ma T, Woods L, Covello A, Hall RC. Keratoconus prevalence among high school students in New Zealand. Cornea. 2019 Nov 1;38(11):1382-9.
- Kim BZ, Meyer JJ, Brookes NH, et al. New Zealand trends in corneal transplantation over the 25 years 1991–2015. Br J Ophthalmol 2017; 101:834-838.
- Angelo L, BopGokul A, McGhee C, Ziaei M. Corneal crosslinking: present and future. The Asia-Pacific Journal of Ophthalmology. 2022 Sep 1;11(5):441-52.
- Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrósio Jr R, Guell JL, Malecaze F, Nishida K, Sangwan VS. Global consensus on keratoconus and ectatic diseases. Cornea. 2015 Apr 1;34(4):359-69.
- Gokul A, Ziaei M, Mathan JJ, Han JV, Misra SL, Patel DV, McGhee CN. The Aotearoa research into keratoconus study: geographic distribution, demographics, and clinical characteristics of keratoconus in New Zealand. Cornea. 2022 Jan 8;41(1):16-22.
- Angelo L, Gokul A, McGhee C, Ziaei M. Comparing Repeatability and Agreement between Commonly Used Corneal Imaging Devices in Keratoconus. Optometry and Vision Science. 2023 Dec 28:10-97.
- Angelo L, Gokul A, McGhee CN, Ziaei M. Keratoconus Management in the Community: A Survey of Optometrists. Eye & Contact Lens. 2024 Jan 1;50(1):10-5.
- Duncan JK, Belin MW, Borgstrom M. Assessing progression of keratoconus: novel tomographic determinants. Eye and Vision. 2016 Dec;3:1-9.
- Meyer JJ, Gokul A, Vellara HR, McGhee CN. Progression of keratoconus in children and adolescents. British Journal of Ophthalmology. 2021 Sep 3.
- Angelo L, Gokul A, Wadhwa H, McGhee CN, Ziaei M. Assessment of Barriers to Accessing a First Specialist Assessment and Follow-up Keratoconus and Crosslinking Service at a Tertiary Referral Centre to Address Health Disparities. Cornea. 2022 May 13:10-97.
- Chan E, Baird PN, Vogrin S, Sundararajan V, Daniell MD, Sahebjada S. Economic impact of keratoconus using a health expenditure questionnaire: a patient perspective. Clinical & Experimental Ophthalmology. 2020 Apr;48(3):287-300.
- Wagner H, Barr JT, Zadnik K, Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: methods and findings to date. Contact Lens and Anterior Eye. 2007 Sep 1;30(4):223-32.

Dr Lize Angelo is a non-training registrar in Hamilton. She submitted her PhD in January this year, supervised by Professor Charles McGhee, Dr Mo Ziaei and Dr Akilesh Gokul, on investigating diagnosis and management of keratoconus, including aspects of equity of access to services in Aotearoa. Her PhD was funded through an HRC Clinical Research Training Fellowship Award.

Dr Akilesh Gokul, a senior lecturer in the University of Auckland’s ophthalmology department, is an optometrist and clinician-scientist focusing on keratoconus, contact lenses, crosslinking and inequity in healthcare. He also works in Te Whatu Ora Auckland’s crosslinking service and at Mortimer Hirst Optometrists as a specialist contact lens clinician.