Dry eye research review 2022
The impact of anticancer drugs on the ocular surface
Chiang et al.
The Ocular Surface, Volume-18, Issue-3, July-2020, Pages 403-417
Review: This article presents a review of published peer-reviewed scientific evidence relating to the side effects of anticancer treatments on the ocular surface and adnexa. It also investigates the possible interactions between coexisting ocular surface disorders and cancer, both requiring treatment with pharmacological drugs.
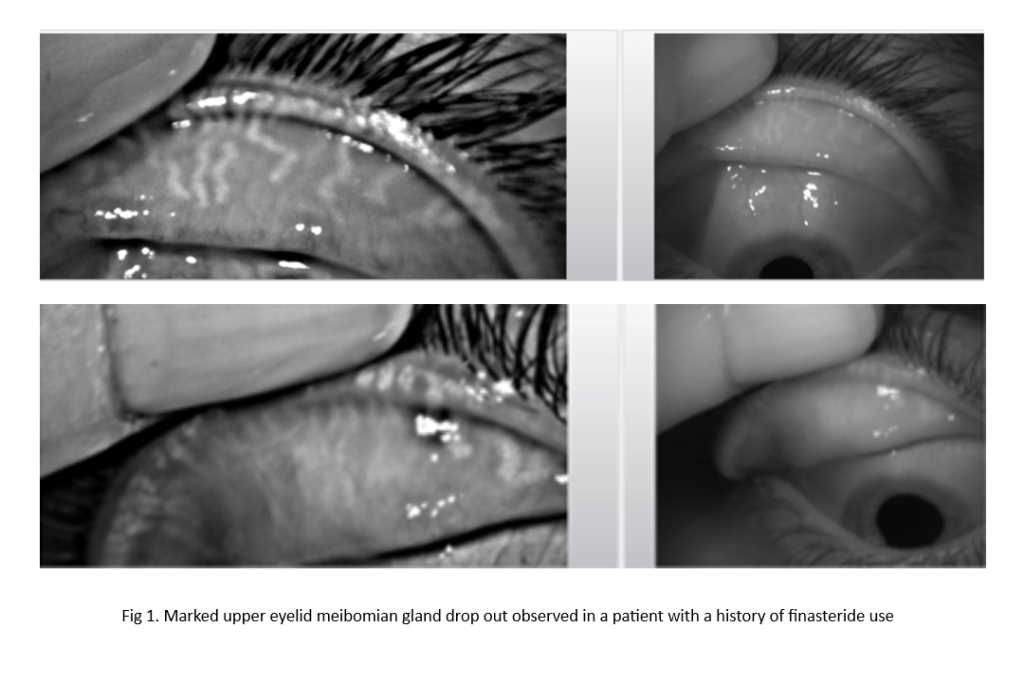
Although the cytotoxic variety of anticancer drugs are formulated to act on rapidly dividing cancerous cells, the drugs can also non-selectively affect other cells undergoing normal cellular growth. Hence, the delicate homeostasis of the ocular surface is also susceptible to chemotoxicity. To maintain homeostasis, the ocular surface relies on an abundance of cells undergoing constant renewal and turnover especially those comprising the skin and hair follicles of the eyelids, conjunctival and corneal epithelial layers, as well as those in the lacrimal apparatus. The impact of the drugs on tear film physiology and biochemistry is also reviewed, with reported ocular symptoms detailed with possible associations with neuropathic pain.
Comment: Anticancer drugs are often not something patients can avoid and because the ocular surface is susceptible to toxicity, due to the ease of disruption in homeostasis of the structures, it is important eyecare practitioners proactively look for ocular side effects in patients who have undergone or are undergoing treatment with anticancer drugs. The potential for interaction between treatment drugs for ocular surface disease and cancer also needs to be considered in practice.
Efficacy and safety of OC-01 nasal spray on signs and symptoms of DED: the ONSET-2 phase 3 randomised trial
Witra et al.
Ophthalmology, Volume-129, Issue-4, April-2022, Pages 379-387
Review: OC-01 (varenicline solution), a pharmacologic neuroactivator of tear film production
used as a nasal spray, has demonstrated potential as a treatment for dry eye disease (DED). This follow-up randomised, double-masked, vehicle-controlled clinical trial further assessed its efficacy and safety.
Adults (n=758) with a pre-existing diagnosis of DED, artificial tear use, Ocular Surface Disease Index score of greater than 23 and Schirmer test score (STS) of 10mm or less participated in the study. While the vector quantity was 50μl for all groups, the concentration of OC-01 dose varied (0.03mg or 0.06mg) between test groups. The primary end point of the study aligned with the US FDA’s cut off value for the approval for drugs for DED, which is an improvement of at least 10mm in STS.
The majority (86.5%) of patients reported at least one treatment-emergent adverse event (TEAE); most were mild and non-ocular (sneezing, cough, throat irritation and instillation site irritation), with fewer from the vehicle-control group, and no serious study drug-related TEAEs reported. A total of five patients (1.9%) in the 0.03 mg group, eight (3.3%) in the 0.06 mg group and four (1.6%) in the vehicle group experienced TEAEs leading to discontinuation.
Comment: As a novel DED treatment approach, the OC-01 nasal spray was well tolerated and showed a clinically meaningful effect on signs and symptoms of DED. Assessment of recommendation likelihood, given the non-conventional approach of applying a DED therapy via the nostrils, would be of interest in fully understanding subjective tolerability of this treatment modality.
Effect of a novel omega-3 and omega-6 fatty acid supplement on DED
Alison Ng et. al.
Optometry and Vision Science: January 2022-Volume 99-Issue 1-p 67-75
Review: This prospective, randomised, double-masked study assessed the effect of the daily consumption of a novel combination of essential fatty acids on the signs and symptoms of dry eye disease (DED).
Fifty participants with moderate-to-severe dry eye participated in this three-month trial involving three study visits at baseline, one month and three months. The Omega-3 Index, evaluated via a finger-prick blood test, was performed to confirm compliance in taking the supplement. The 24 participants randomised to the treatment group received a combination of omega-3 and omega-6 fatty acids while the control group received a combination of coconut oil and olive oil. Although both groups showed improvement in OSDI scores relative to baseline, the treatment group improved more than the control group. The Omega-3 Index increased by 34% in the treatment group with no change in the placebo group, confirming a high level of compliance. No significant differences were found between any other ocular clinical parameters assessed between treatment and control groups.
Comment: Objective confirmation of compliance in taking the study formulation is a strongpoint of the research design. Together with the double-masked nature of the study, it lends confidence in the outcomes that show symptomatic relief is achievable for patients with moderate-to-severe dry eye who take the essential fatty acid combination as described in this study.

Dr Kalika Bandamwar recently joined the University of Auckland’s Ocular Surface Laboratory as a research fellow. Her research interests include dry eye, ocular surface and contact lenses. She completed her PhD under the supervision of Professor Eric Papas at the University of New South Wales in 2012.