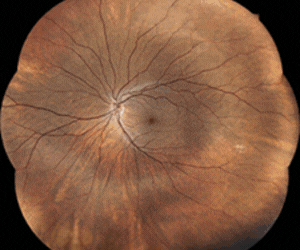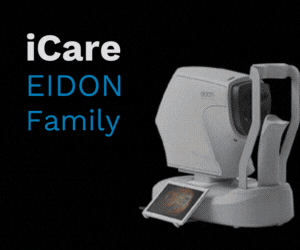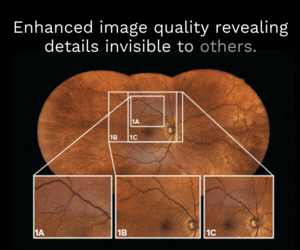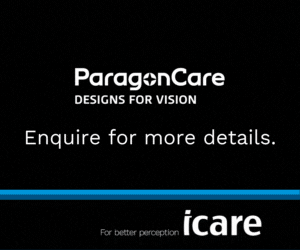
Atropine mist trial underway
The State University of New York (SUNY) College of Optometry is the latest research centre to join Eyenovia’s multi-centre clinical trial into the safety and efficacy of its investigational drug, MicroPine, developed to help slow myopic progression in children aged three to 12.
MicroPine is an atropine eye solution formulated for administration as a mist using Eyenovia’s patented microdose dispenser.
“I believe some of the current challenges faced by clinicians and families exploring myopia control with atropine are adherence and side effects with long-term treatment,” said Assistant Professor Danielle Iacono, SUNY’s lead investigator. "The Eyenovia micro-dosed atropine formulations to be evaluated in the study are about one quarter the volume of an eyedrop. This means kids receive less drug, which could make treatment better tolerated and increase adherence to treatment.”
Eyenovia’s Chaperone study is a US-based, randomised, double-masked trial of more than 400 children. Subjects will be randomised to receive treatment with either of two MicroPine concentrations or a placebo over four years. The primary endpoint of the study is the change in refractive error from baseline through to 36 months. All children will receive MicroPine for at least one of the four years of the study and some children may receive MicroPine for all four years.