Diagnosis and management of macular polypoidal choroidal vasculopathy
Polypoidal choroidal vasculopathy (PCV) has historically been considered a subtype of neovascular age-related macular degeneration (nAMD). It is characterised by recurrent serosanguinous maculopathy, in which type 1 macular neovascularisation is associated with an abnormal branching network of vessels, along with aneurysmal dilations (polypoidal lesions) located under the retinal pigment epithelium (RPE). Early identification of this type of macular neovascularisation is important since it has implications for patient management.
Epidemiology, risk factors and pathogenesis
In patients with nAMD, the PCV subtype is more prevalent in patients of Asian (22-55%) and Polynesian heritage, presenting as unilateral neovascularisation at the macula1. This is in contrast to Caucasian patients (8-25%), where bilateral findings and peripapillary neovascularisation is more common2. PCV can also manifest outside the posterior vascular arcade as peripheral exudative haemorrhagic chorioretinopathy.
Systemic risk factors include those typical for nAMD: cigarette smoking, higher body mass index and elevated serum c-reactive protein. Pachychoroid features observed in eyes with PCV – which is characterised by focal choroidal thickening attributed to pathologically dilated outer choroidal vessels (Haller’s layer) – lead to secondary focal attenuation of the choriocapillaris. Topographically, these changes correlate to the branching vascular network (BVN) ingrowth visualised on enhanced depth-imaging optical coherence tomography (OCT), and may underlie the pathogenesis of neovascular complexes seen in eyes with PCV3.
Clinical examination and multimodal imaging
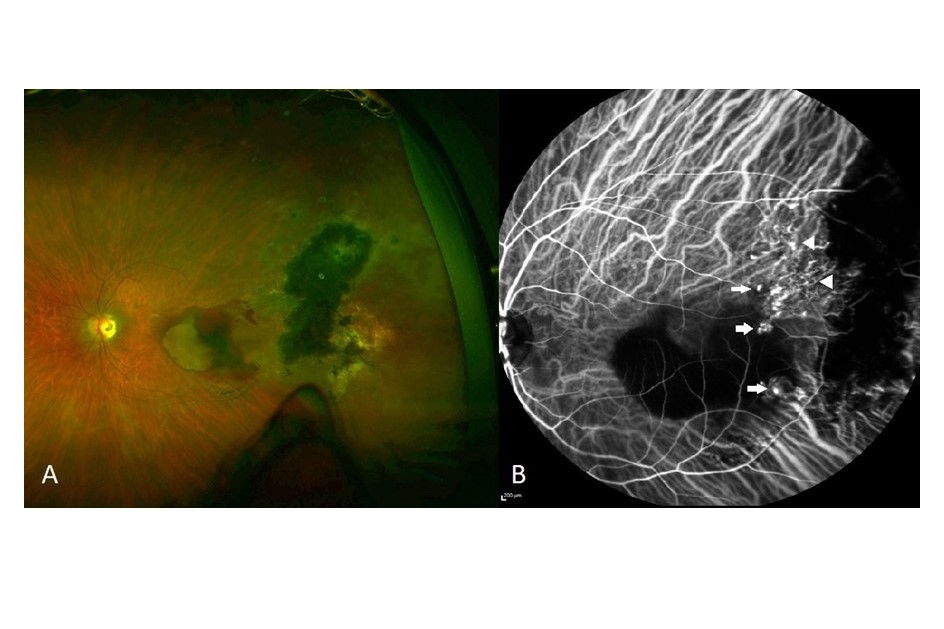
PCV is characterised by reddish-orange nodules on fundoscopy and can be associated with extensive subretinal and sub-RPE haemorrhage (Fig 1A). In contrast to typical nAMD, soft drusen and geographic atrophy are usually absent.
Multimodal imaging has proved extremely useful in the diagnosis and management of PCV. Fluorescein angiography (FA) provides a good measure of disease activity; however, sub-RPE structures are poorly visualised. This limitation is overcome with indocyanine green angiography (ICGA), with the characteristic findings of single or multiple polypoidal lesions visible in the early phases of the angiogram (Fig 1B), along with several additional features listed in Table 14. Although ICGA is the gold standard imaging modality to confirm PCV, the procedure is invasive, time-consuming and is not widely accessible or routinely required in all patients with nAMD.
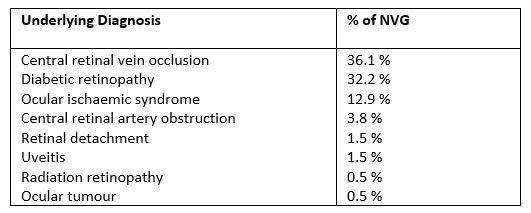
Table 1. PCV diagnostic criteria used in the EVEREST II trial.
Structural domain OCT (SD-OCT) is non-invasive and has become the mainstay imaging method for diagnosing and managing macular diseases. The Asia-Pacific Ocular Imaging Society PCV Workgroup evaluated a set of diagnostic criteria using a combination of non-ICG-based imaging with high accuracy (Table 2). Although many of the listed findings are not exclusive to PCV, a combination of three features including en-face OCT complex RPE elevation, sharp peaked pigment epithelial detachment (PED), and sub-RPE ring-like lesion achieved good sensitivity (75%) and high specificity (91%) with an area under the receiver operating characteristic curve of 0.9 (Fig 2). This was validated on an independent dataset of 80 eyes (using ICG confirmation) with 82% accuracy5.
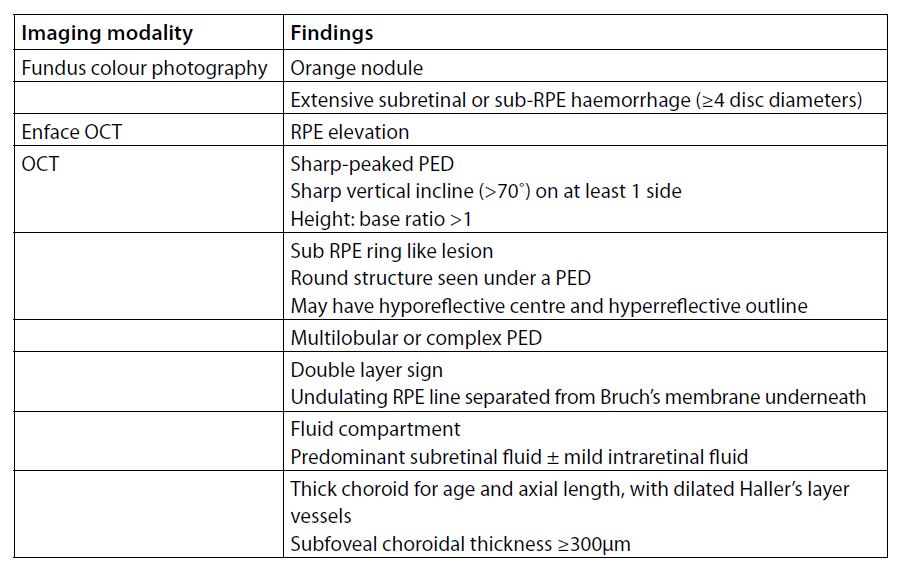
Table 2. Imaging features observed in PCV.
OCT-angiography (OCT-A) is gaining popularity as a non-invasive alternative to conventional angiography. Takayama et al found OCT-A detected more branching vascular networks but fewer polypoidal lesions than ICGA, possibly because of the variable flow rate through polypoidal structures6. At present, OCT-A cannot replace ICGA in the diagnosis of PCV.
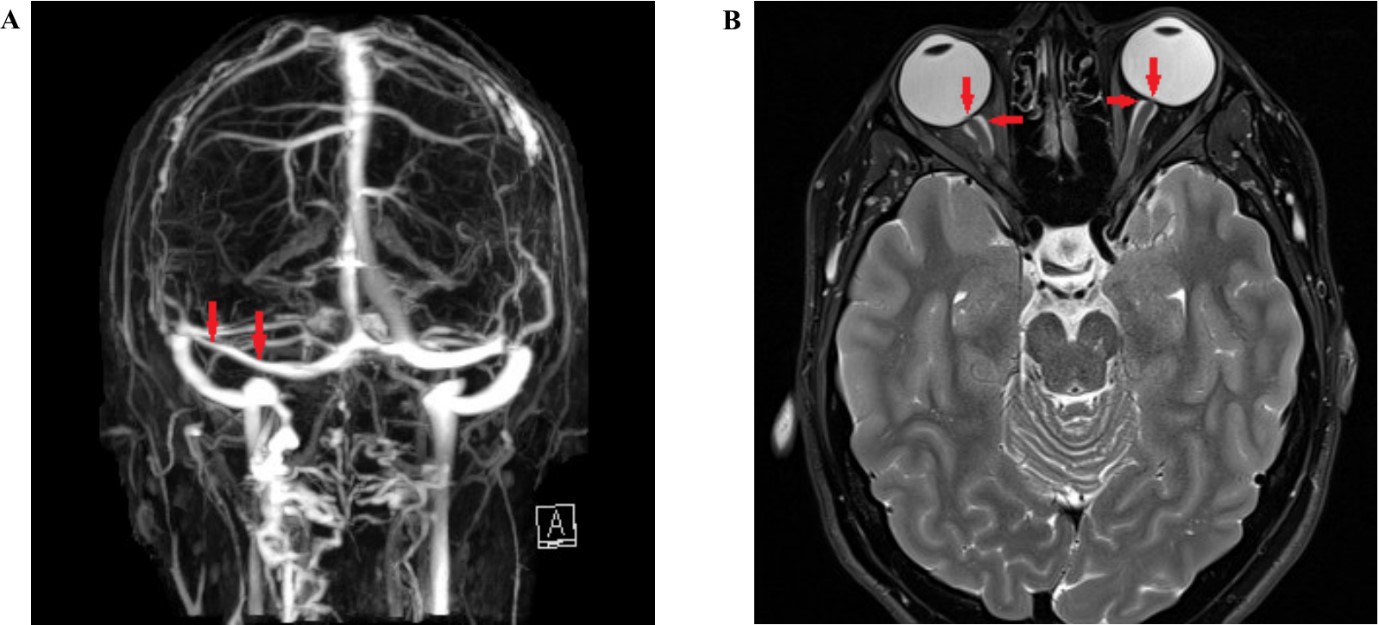
Fig 2. (A) 65-year-old East-Asian male with recurrent serosanguinous maculopathy in the right eye. (B) ICGA demonstrating a branching network of vessels and a cluster of polyps at the superior macula. (C) RPE elevation observed on en-face OCT (arrow heads). (D) A multilobular PED on cross-sectional OCT of the superior macula, including a sharp peaked PED with a round sub-RPE ring like lesion (arrow) corresponding to one of the many polypoidal lesions seen on ICGA. (E) Double layered sign characterised by two hyper-reflective lines (arrow heads) with the RPE layer above and Bruch’s membrane. A separate sub-RPE ring like lesion is also visible (arrow)
Management
Although spontaneous closure of polypoidal lesions can occur, loss in best-corrected visual acuity (BCVA) occurs in most untreated cases because of worsening haemorrhage, exudation and subretinal fibrosis. Laser photocoagulation is not appropriate for macula lesions because of concerns of scarring and disease recurrence.
Several prospective studies have compared intravitreal anti-VEGF therapy and photodynamic therapy (PDT) and their effect on visual acuity, disease activity and regression of polypoid lesions in patients with PCV. PDT utilises the photosensitive drug verteporfin (Visudyne) in combination with a long-duration infrared laser, which causes thrombosis and vascular damage. Closure of polypoidal lesions using standard dose-fluence PDT has been reported as high as 94%. A meta-analysis of 316 eyes showed modest improvements in visual acuity in the first 18 months, returning to baseline (or worse) beyond two years. Despite achieving closure of polypoidal lesions, BVN can persist and PDT treatments usually need to be repeated. Cumulative recurrences and development of new polypoidal lesions was noted in 53% at three years, with recurrences more likely to experience ≥3 lines loss in vision7. Other concerns include the risk of worsening exudation, occult haemorrhage and irreversible damage to the choriocapillaris and RPE from repeated treatments.
In contrast to PDT, intravitreal anti-VEGF therapies work by reducing exudation and vascular leakage but are less effective at achieving closure of polypoidal lesions. The Everest study, a randomised trial performed across multiple Asian centres, showed PDT monotherapy (78%) or ranibizumab (Lucentis) + standard dose-fluence PDT combination therapy (71%) achieved higher rates of polypoidal lesion closure compared to ranibizumab monotherapy (29%). The effects of the treatment modalities on vision were answered in Everest II, which compared ranibizumab monotherapy to the combination therapy. At 12 months, patients receiving combination therapy experienced a greater mean improvement in BCVA (8.3 vs 5.1 letters p=0.01), higher rates of lesion regression (69% vs. 35% p < 0.001) and required fewer injections (mean 4.0 vs 7.0), and this persisted at 24 months8. The Fujisan study examined the role of early versus deferred PDT in combination with ranibizumab and found similar visual and anatomical outcomes between the two arms, however the early PDT arm required significantly fewer injections10.
Another study, the Planet study investigated the effect of fixed aflibercept (Eylea or Zaltrap) with or without rescue PDT (standard dose-fluence). At 52 weeks, those receiving fixed dosing aflibercept monotherapy were non-inferior to the combined group with a mean gain of 10.7 letters of vision. Polyp regression was achieved in 39% and more than 80% showed no signs of polyp activity at 12 months in the aflibercept monotherapy arm, with no advantage of rescue PDT up to 96 weeks follow up. With only 15% meeting rescue criteria, the study was underpowered to show any benefit of combination therapy11. Unpublished data from the Atlantic study supported Planet’s findings, with no additional benefit of rescue PDT in patients receiving aflibercept monotherapy (treat and extend dosing) regarding gain in visual acuity or polyp activity compared to aflibercept monotherapy in Caucasian patients with PCV12.
Real-world outcomes from the Fight Retinal Blindness registry, which included patients treated in New Zealand, reported a greater mean improvement in vision (+16.9 letter vs 8.2 letters), quicker time to inactivity and fewer injections in patients receiving combination compared with anti-VEGF monotherapy. In contrast to randomised trials, however, most patients (66%) received bevacizumab (Avastin)13. Although there are no head-to-head prospective trials comparing the three anti-VEGF agents, a comparative retrospective study showed the rate of polyp regression was significantly higher in patients receiving aflibercept compared with the ranibizumab group, though there was no difference in visual outcome at 12 months14. The higher closure rate with aflibercept might be attributed to a longer half-life and a higher affinity for VEGF-A compared with ranibizumab, as well as its ability to inhibit VEGF-B and placental growth factor.
Practical considerations
PCV should be considered in all patients with macular neovascularisation/nAMD, especially in those of Asian or Polynesian heritage. Aflibercept monotherapy or combination therapy (ranibizumab/bevacizumab + PDT) are both effective for controlling disease activity and improving vision. The significance of a higher polyp closure rate with combination therapy is still unclear.
In New Zealand, patients must show persistent disease activity despite three initial bevacizumab injections before being eligible to receive aflibercept. Although the vast majority of patients respond favourably to anti-VEGF monotherapy, considerable heterogeneity in treatment response and outcomes occur, attributed to differences in patient demographics and baseline characteristics, including PCV subtype2,15. For this reason, initial or rescue PDT still has an important role in management. Referral to centres with access to ICGA and PDT should be considered for those with persistent clinical activity despite anti-VEGF therapy, or to reduce the treatment burden for those where extension of injection interval is not being achieved.
References
- Wang N, Squirrell D. To characterise polyp and abnormal vascular network morphology and location using indocyanine green (ICG) angiography in a diverse Auckland patient population. Abstract presented RANZCO NZ Branch Meeting, 19 May 2021.
- Imamura Y, Engelbert M, Iida T et al. PCV: A Review. Survey of Ophthalmology. 2010;55:501-15.
- Balaratnasingam C, Lee WK, Koizumi H, et al. PCV: a distinct disease or manifestation of many? Retina. 2016;36:1-8.
- Cheung CMG, Lai TYY, Teo K et al. PCV: consensus nomenclature and non-indocyanine green angiograph diagnostic criteria from the Asia-Pacific Ocular Imaging Society PCV Workgroup. Ophthalmology. 2021;128(3):443-452.
- Cheung CMG, Lai TYY, Ruamviboonsuk P, et al. PCV: definition, pathogenesis, diagnosis and management. Ophthalmology. 2018;125(5)708-724.
- Takayama K, Ito Y, Kaneko H, et al. Comparison of indocyanine green angiography and optical coherence tomographic angiography in PCV. Eye. 2017; 31(1):45-52.
- Wong CW, Cheung CMG, Mathur R, et al. Three-year results of PCV treatmed with photodynamic therapy. Retina. 2015;35(8):1577-93.
- Koh A, Lee WK, Chen LJ, et al. Everest study: Efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular PCV. Retina. 2012;32(8):1453-64
- Lim TH, Lai TYY, Takahashi K, et al. Comparison of ranibizumab with or without verteporfin photodynamic therapy for PCV: The EVEREST II randomized clinical trial. JAMA Ophthalmology. 2020;138(9);935-942.
- Gomi F, MD P, Oshima Y, et al. Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of PCV: the Fujisan study. Retina. 2015; 35(8):1569-76.
- Wong TY, Ogura Y, Lee WK et al; Planet investigators. Efficacy and safety of intravitreal aflibercept for PCV: two-year results of the aflibercept in PCV study. American Journal of Ophthalmology. 2019;204:80-89.
- Marques JP, Farinha C, Costa MÂ et al. Protocol for a randomised, double-masked, sham-controlled phase 4 study on the efficacy, safety and tolerability of intravitreal aflibercept monotherapy compared with aflibercept with adjunctive photodynamic therapy in PCV: the Atlantic study. BMJ Open. 2017;28;7(8):e015785.
- Chong Teo KY, Squirrell DM, Nguyen V, et al. A multicountry comparison of real-world management and outcomes of PCV: Fight Retinal Blindness! cohort. Ophthalmology Retina. 2019;3(3):220-229.
- Cho HJ, Kim KM, Kim HS, et al. Intravitreal aflibercept and ranibizumab injections for PCV. American Journal of Ophthalmology. 2016;165:1-6.
- Cheung CMG, Tan CS, Patalauskaite R et al. Ranibizumab with or without verteporfin photodynamic therapy for PCV: predictors of visual and anatomical response in the EVEREST II study. Retina. 2021 Feb 1;41(2):387-392

Dr Riyaz Bhikoo is the new William Ross surgical retina fellow, following his fellowship in ocular oncology and medical retina at the University of British Columbia in Vancouver, Canada. He moved to UBC following an 18-month vitreoretinal fellowship in Perth, Australia, after completing his vocational training in New Zealand. He plans to return to New Zealand in 2022.