Faricimab – UK approval
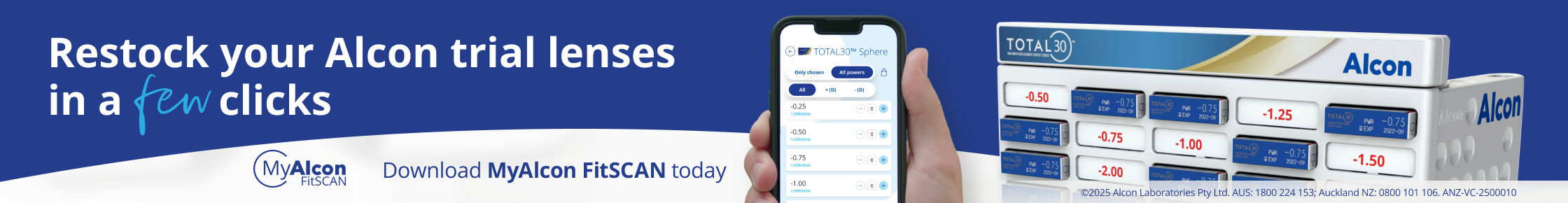
The UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) has approved faricimab (Roche, Vabysmo), the first bispecific antibody to treat neovascular age-related macular degeneration (nAMD) and diabetic macular oedema (DMO), for NHS patients in England.
Cathy Yelf, chief executive of UK sight loss charity the Macular Society, said patients with nAMD and DMO face regular hospital visits to receive treatment to save their sight. “We know these trips can be arduous and often rely on the support of friends and family, sometimes as often as every four weeks. So we are delighted that a new treatment option, which has the potential to maintain vision and help minimise the number of hospital visits, will be made available to patients.”
The NHS deal follows faricimab’s January 2022 approval by the FDA. In Australia the Pharmaceutical Benefits Advisory Committee (PBAC) confirmed it is evaluating the new anti-VEGF drug, while the Australian arm of the multicentre Rhine faricimab trial is currently recruiting patients. Roche also sought Australian Pharmaceutical Benefits Scheme listing of the drug to treat DMO at May 2022’s PBAC meeting.
In New Zealand, Medsafe’s Medicine Classifications Committee recommended faricimab be added to the country’s prescription medicine schedule on 26 October 2021, but at the time of writing it was not yet approved. “We would welcome a funding application to consider including faricimab on the Pharmaceutical Schedule once approved by Medsafe,” said Rosa Bach, Pharmac’s communications advisor.
In phase-3 clinical trials, reported in The Lancet in February this year, nAMD patients given faricimab at up to 16-week intervals had similar visual outcomes to those given aflibercept every eight weeks. “These data reinforce the potential of faricimab as an important treatment option that may help improve and maintain vision while extending the time between treatments up to four months,” said Dr Levi Garraway, Roche’s chief medical officer.