IPL therapy for MGD
Outcomes of a randomised controlled trial
By Dr Ally Xue, Dr Michael Wang, Dr Sue Ormonde and A/Prof Jennifer Craig
Evaporative Dry Eye
Evaporative disease is acknowledged to be the most prevalent dry eye subtype.(1) It is commonly caused by meibomian gland dysfunction (MGD), where the increased viscosity of meibum can lead to blockage and inflammation of the ductal system.(2,3) The resulting reduction in tear film lipid layer quality can then precipitate a vicious cycle of tear film instability, hyper-evaporation, hyperosmolarity, and inflammation.(2,4)
Evaporative dry eye (EDE) causes symptoms of ocular discomfort and visual impairment that can severely impact on a patient’s quality of life.(1,5-7) However, self-management compliance with current standard MGD management strategies (such as artificial tears, warm compress therapy and eyelid hygiene regimen) is difficult to sustain due to their time consuming nature and perceived transient effects.(8,9) This presents an ongoing challenge to patients and clinicians and highlights a need for the development of alternative management options.(8)
Intense pulsed light
One novel dry eye treatment to emerge in recent years is intense pulsed light (IPL) therapy. IPL has traditionally been used in the cosmetic industry for the treatment of a variety of dermatological conditions.(10) IPL devices release high-intensity polychromatic light, ranging from the infrared to visible spectrum, and the radiation can be adjusted for targeted delivery of thermal energy to selected structures.(10) There has been growing interest in the role of IPL technology as a potential treatment for MGD,(11-20) following unanticipated accounts of improved tear film function after IPL treatment for rosacea.(21) Although numerous studies have reported improvements in ocular surface signs and symptoms following IPL therapy,(11-20) more research is required to facilitate a greater understanding of its mechanisms of action and long-term treatment profile.(21)
The growing commercial popularity of IPL as a dry eye treatment prompted the Ocular Surface Laboratory at The University of Auckland to design and conduct a doublemasked, randomised controlled trial to further characterise the longer-term cumulative treatment effects of IPL therapy in MGD patients.
IPL study design
Eighty-seven participants (67% female, 33% male; mean±SD age, 53±16 years) with dry eye symptoms (22) and clinical signs of MGD(23,24) were recruited in a double-blinded, randomised controlled trial. They were randomly assigned to one of three treatment groups; intense regulated pulsed light (IRPL) therapy (E-Eye; E-Swin) with either four or five applications (flashes) of homogenously sequenced light pulses, or placebo (sham) treatment (Figs 1-3).
Treatments were applied to both eyes, during clinic visits on days 0, 15, 45, and 75. With the globes protected, light pulses were delivered to periocular zones inferior to each eye (Figs 2, 3), and the pulse intensity ranged from 9 to 13 J/ cm2 according to the participant’s Fitzpatrick skin phototype.(20) Participants allocated to the placebo group underwent sham treatment, with a non-illuminating handpiece applied to the periocular area, while an active headpiece of an identical device was directed towards the corner of the room, away from the patient, to mimic the illumination and sounds of the IPL device. Importantly, in this study, meibum expression was not performed immediately after application, as it might in a traditional clinical setting, in order to isolate the effects of IPL therapy.
Dry eye symptoms, tear film quality, and ocular surface health were assessed immediately before treatment on days 0, 15, 45, 75, and four weeks following the treatment course completion on day 105. Symptoms were assessed with the Ocular Surface Disease Index (OSDI), the Standardised Patient Evaluation of Eye Dryness (SPEED) questionnaire and the Symptom Assessment iN Dry Eye (SANDE) visual analogue scale. Non-invasive tear film breakup time, tear film lipid layer grade, tear meniscus height, bulbar conjunctival hyperaemia and infrared meibography were assessed using the Oculus Keratograph 5M. Tear film osmolarity was measured with a clinical osmometer (TearLab).(22)
Lid margin, eyelash abnormalities and lid parallel conjunctival folds (LIPCOF) were graded by slit lamp biomicroscopy examination. Ocular surface evaluation was performed by assessment of the corneal, conjunctival and lid wiper epithelia with the aid of sodium fluorescein and lissamine green dyes. Meibum quality and meibomian gland expressibility of the inferior eyelid was assessed using the Meibomian Gland Evaluator (TearScience, Johnson & Johnson).
Conjunctival expression markers of ocular surface inflammation and goblet cell function, as well as eyelid swabs for microbial cultures were collected, and lashes for Demodex mite evaluation epilated, at baseline and day 105.
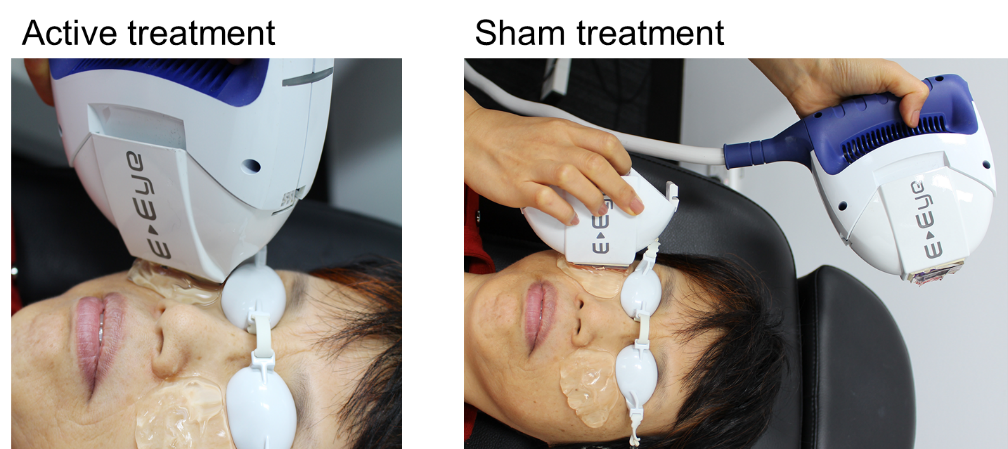
Fig 2. IPL application during active (A) and sham (B) treatment
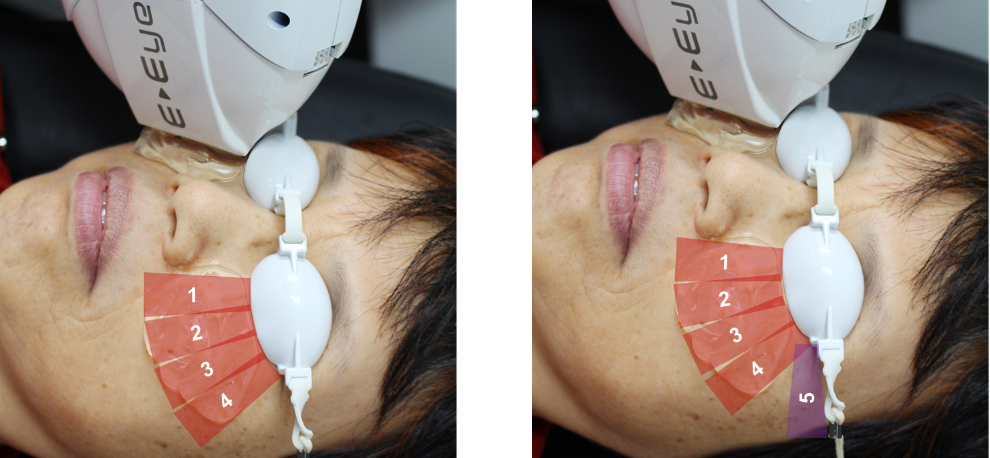
Fig 3. IPL application during four flashes (left) and five flashes (right) treatment
IPL study results
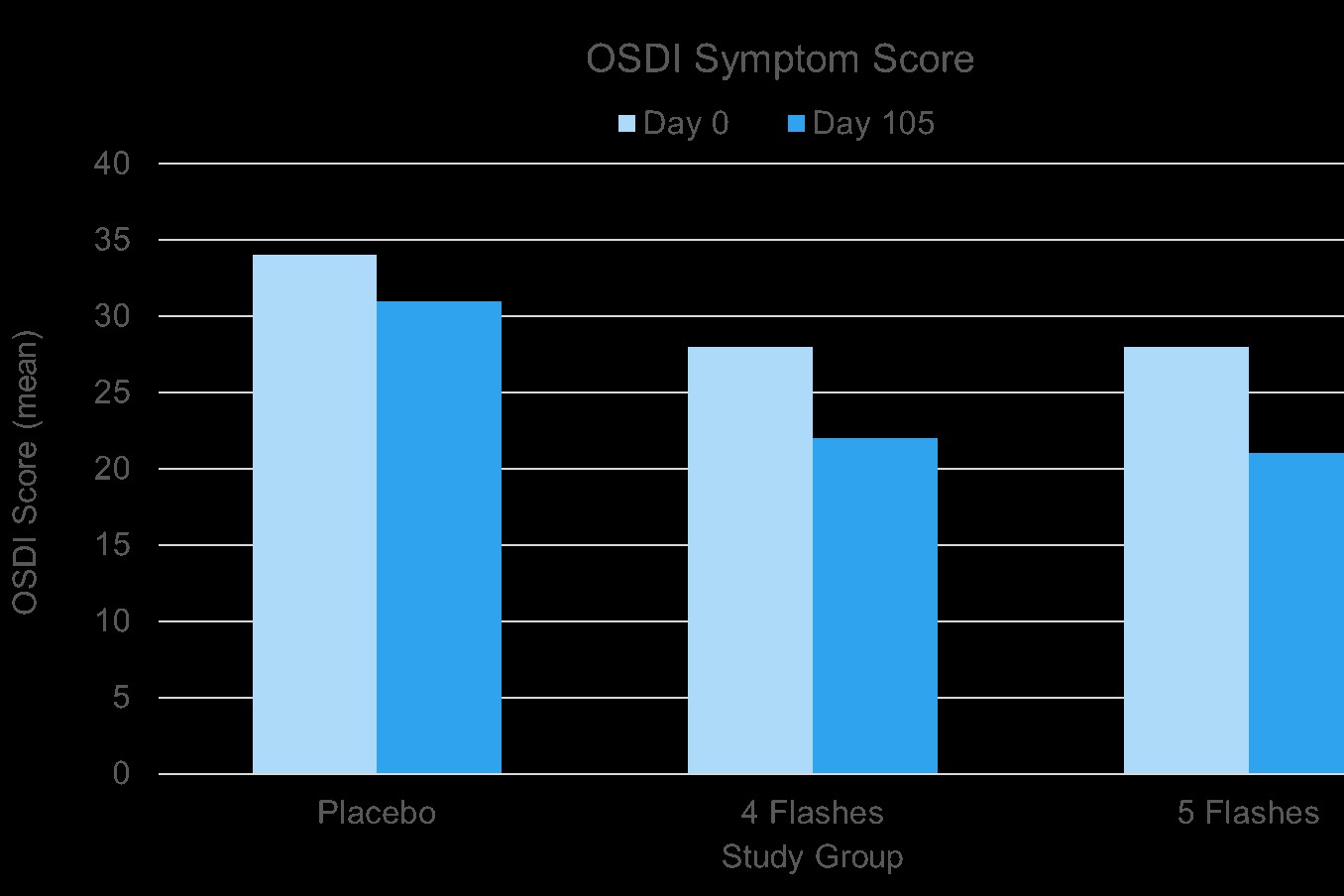
There were no significant differences in clinical measurements between treatment groups at baseline (all p>0.05). Participants allocated to four flashes of IPL demonstrated greater reductions in OSDI, SPEED, and SANDE scores relative to the placebo group from day 75 onwards (all p<0.05) (Fig 4). Participants in the five-flash IPL group exhibited improvements in OSDI and SANDE scores from day 15 onwards, although consistent reductions in the SPEED score did not occur until day 75 onwards (all p<0.05). Symptomatic improvements were also accompanied by a decrease in severity of meibomian gland capping for both IPL treatment groups on day 105, although improvements on day 45 were observed only in the five-flash IPL group (all p<0.05). Furthermore, there was an increase in tear film lipid layer grade in the four-flash IPL group from day 75 onwards, while improvements in the five-flash IPL group were observed from day 45 onwards (all p<0.05). Interestingly, inhibition of Corynebacterium macginleyi growth was also detected in both treatment groups during the study period (all p<0.05), but no reduction in Demodex mite count was observed.
IPL study conclusions
Consistent with the findings of previous studies,(11-20) this randomised trial demonstrated the clinical efficacy of IPL therapy in the management of MGD. Significant improvements in clinical signs and symptoms of ocular surface disease were observed during the four-month period in both treatment groups, with significant reductions in dry eye questionnaire scores (Fig 4), which was associated with enhanced tear lipid layer quality and diminished meibomian gland capping.
Moreover, inhibition in the growth of some bacteria was detected following the IPL treatment course. The results also showed that five-flash IPL therapy has greater treatment efficacy than four-flash IPL therapy, with earlier improvements in dry eye symptom scores on day 15 in participants receiving five flashes. Similarly, on day 45, improvements in OSDI and SANDE symptomology scores, tear lipid layer quality, and meibomian gland capping, were only observed in participants randomised to the fiveflash IPL therapy group.
Based on trends observed in the results, it appears that, to allow for sustained cumulative treatment effects to be achieved, recommending a preliminary course of four treatments is advisable before making a clinical decision about overall therapeutic efficacy for an individual patient.
The deliberate omission of mechanical gland expression post-IPL, in order to isolate the effects of the IPL, undoubtedly lessened the likelihood of observing more significant improvements in other MGD signs (such as non-invasive tear film stability) reported elsewhere.(12-17,19,20) In addition, outcome measures in this study were collected up to four weeks after the treatment was applied in each case, which means any immediate or transient therapeutic effects, especially in inherently variable parameters such as tear film stability, would be missed.(22,25) Future studies with more extended courses of IPL therapy are required to investigate whether longer treatment courses might offer additional clinical efficacy.
The mechanisms of action of IPL therapy in the treatment of MGD remains yet to be fully understood, but numerous hypotheses have been proposed over the years.(11,13,18,21,26) A key understanding is that thermal energy delivered by IPL therapy liquefies the viscous meibum observed in MGD, thereby encouraging the release of meibum into the tear film.(8,9,11,21,26) It is recognised from clinical studies that an intact lipid layer is necessary to prevent excessive aqueous evaporation from the tear film,(27) and this hypothesis would appear to be supported by the findings of the current study, which demonstrate enhanced tear film lipid layer quality and reduced meibomian gland capping following treatment. Another hypothesis suggests that IPL therapy may decrease and/ or alter the composition of the periocular microbiota and contribute to reduction of ocular surface inflammation.(21,26) A decrease in Corynebacterium macginleyi levels was observed following IPL therapy in the current study. Treatment effects of IPL therapy have also been hypothsised to be mediated by the reduction in ocular Demodex load, reduction of epithelial turnover and thrombosis of abnormal blood vessels in the periocular skin, downregulation of inflammatory cascades and modulation of the levels of reactive oxidative species.(13,18,26,28) However, evidence supporting these hypotheses was not able to be confirmed in the current trial.
The scientific evidence presented in this study confirms lasting improvement in symptom relief and clinical signs of MGD following a course of IPL treatments. This research provides reassurance for eye care professionals that IPL offers a safe and overall effective treatment option for tackling evaporative dry eye in patients affected by MGD.
Acknowledgements
The authors gratefully acknowledge the assistance of clinical researchers of the Ocular Surface Laboratory, past and present, who have been involved in the research over the last five years, especially Drs Sanjay Marasini, Isabella Cheung, Joevy Lim, Ji Soo Kim and Mr Sang Hoo Lee. The authors also appreciate grant support from NZOVRF and unrestricted funding from E-Swin, for this investigator-led trial.
References
1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276-283.
2. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II Pathophysiology report. Ocul Surf. 2017;15(3):438-510.
3. Knop E, Knop N, Millar T, Obata H, Sullivan DA. The International Workshop on Meibomian Gland Dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938-1978.
4. Baudouin C, Messmer EM, Aragona P, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100(3):300-306.
5. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334-365.
6. Belmonte C, Nichols JJ, Cox SM, et al. TFOS DEWS II Pain and Sensation report. Ocul Surf. 2017;15(3):404-437.
7. Mathews PM, Ramulu PY, Swenor BS, Utine CA, Rubin GS, Akpek EK. Functional impairment of reading in patients with dry eye. Br J Ophthalmol. 2017;101(4):481-486.
8. Jones L, Downie LE, Korb D, et al. TFOS DEWS II Management and Therapy Report. Ocul Surf. 2017;15(3):575-628.
9. Geerling G, Tauber J, Baudouin C, et al. The International Workshop on Meibomian Gland Dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050-2064.
10. Wat H, Wu DC, Rao J, Goldman MP. Application of intense pulsed light in the treatment of dermatologic disease: a systematic review. Dermatol Surg. 2014;40(4):359-377.
11. Ahmed SA, Taher IME, Ghoneim DF, Safwat AEM. Effect of intense iulsed light therapy on tear proteins and lipids in meibomian gland dysfunction. J Ophthalmic Vis Res. 2019;14(1):3-10.
12. Arita R, Fukuoka S, Morishige N. Therapeutic efficacy of intense pulsed light in patients with refractory meibomian gland dysfunction. Ocul Surf. 2019;17(1):104-110.
13. Choi M, Han SJ, Ji YW, et al. Meibum expressibility improvement as a therapeutic target of intense pulsed light treatment in meibomian gland dysfunction and its association with tear inflammatory cytokines. Sci Rep. 2019;9(1):7648.
14. Li D, Lin SB, Cheng B. Intense pulsed light treatment for meibomian gland dysfunction in skin types III/IV. Photobiomodul Photomed Laser Surg. 2019;37(2):70-76.
15. Vigo L, Giannaccare G, Sebastiani S, Pellegrini M, Carones F. Intense pulsed light for the treatment of dry eye owing to meibomian gland dysfunction. J Vis Exp: JoVE. 2019(146).
16. Albietz JM, Schmid KL. Intense pulsed light treatment and meibomian gland expression for moderate to advanced meibomian gland dysfunction. Clin Exp Optom. 2018;101(1):23-33.
17. Rong B, Tang Y, Liu R, et al. Long-term effects of intense pulsed light combined with meibomian gland expression in the treatment of meibomian gland dysfunction. Photomed Laser Surg. 2018;36(10):562-567.
18. Liu R, Rong B, Tu P, et al. Analysis of cytokine levels in tears and clinical correlations after intense pulsed light treating meibomian gland dysfunction. Am J Ophthalmol. 2017;183:81-90.
19. Jiang X, Lv H, Song H, et al. Evaluation of the safety and effectiveness of intense pulsed light in the treatment of meibomian gland dysfunction. J Ophthalmol. 2016;2016:1910694.
20. Craig JP, Chen YH, Turnbull PR. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2015;56(3):1965-1970.
21. Vora GK, Gupta PK. Intense pulsed light therapy for the treatment of evaporative dry eye disease. Curr Opin Ophthalmol. 2015;26(4):314-318.
22. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology Report. Ocul Surf. 2017;15(3):539-574.
23. Tomlinson A, Bron AJ, Korb DR, et al. The International Workshop on Meibomian Gland Dysfunction. 2011;52(4):2006-2049.
24. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1(3):107- 126.
25. Sullivan BD, Crews LA, Sonmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012;31(9):1000-1008.
26. Dell SJ. Intense pulsed light for evaporative dry eye disease. Clin Ophthalmol. 2017;11:1167-1173.
27. Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci. 1997;74(1):8-13. 28. Zhang X, Song N, Gong L. Therapeutic effect of intense pulsed light on ocular demodicosis. Curr Eye Res. 2019;44(3):250-256.