Is UGH syndrome still relevant in 2020?
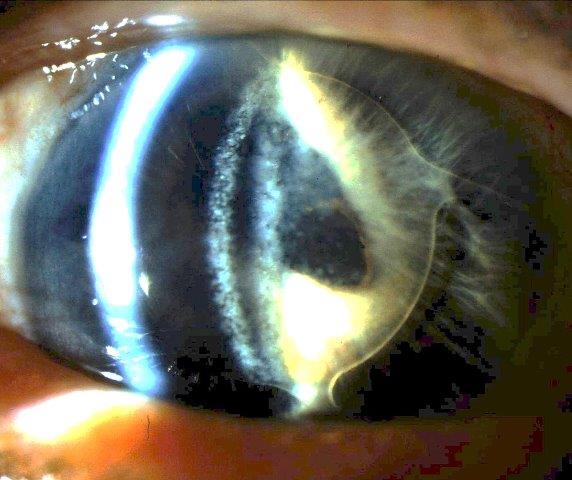
Uveitis-glaucoma-hyphaema (UGH) syndrome is a late, postoperative complication traditionally associated with implantation of anterior chamber (AC) intraocular lenses (IOLs) following cataract surgery (Fig 1)¹. However, it can also be caused by contemporary AC-IOLs, other IOLs (Fig 2) and other anterior segment devices¹.
It is characterised by chronic or recurrent anterior segment inflammation, elevated intraocular pressure (IOP) and intraocular haemorrhage¹. If left untreated, it can progress to cystoid macula oedema (CMO) and glaucomatous optic neuropathy leading to a loss of vision. UGH syndrome as an indication of IOL exchanges or removals comprised 9-16% of all cases in the 1990s, and still comprises 7.5-12% in recent studies¹. Therefore, although it is generally agreed the rates of UGH syndrome have decreased with improved IOL designs and materials, it still remains very relevant today¹.
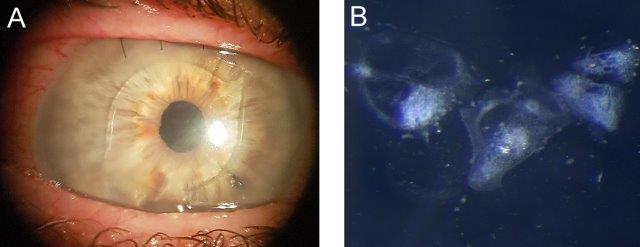
Fig 2. A) Pre-op image showing fine keratic precipitates on the IOL and early corneal decompensation; B) High magnification stereo-microscope image of the IOL under side illumination showing the appearance of keratic precipitates
Aetiology
UGH syndrome is caused by chaffing or mechanical irritation by intraocular implants against the iris1,2. When first described in 1978, it was associated with the use of rigid AC-IOLs and warping of the footplate leading to iris and iridocorneal angle chaffing, which typically developed within a year of implantation⁴. Cases in the 1990s were also commonly caused by iris-fixated IOLs and, less commonly, posterior chamber IOLs¹.
In the last decade, sulcus IOLs have become the most common cause, especially when a single piece acrylic lens or a square-edge optic lens is used (Fig 3)¹. Other causes include scleral-fixated IOLs, iris implants, endocapsular tension rings and glaucoma filtration devices¹. In the bag IOLs are a more rare cause¹. Prior history of ocular trauma, pars plana vitrectomy, pseudoexfoliation syndrome, connective tissue disorders or surgical complications during cataract surgery are possible predispositions.
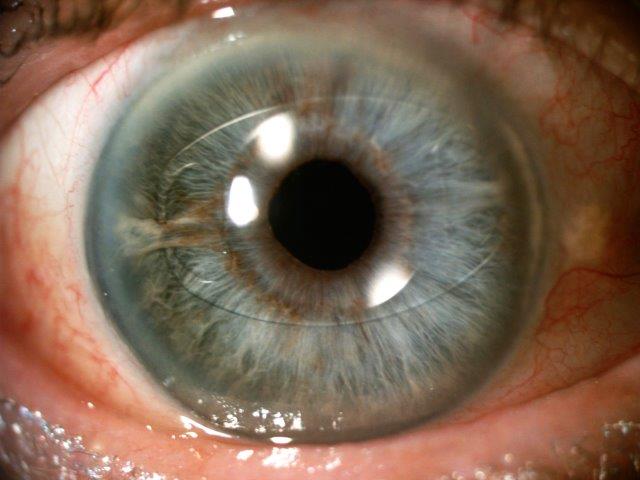
Fig 3. Long-term UGH syndrome, with VA 6/36 pre-op. Treated by exchange of ciliary sulcus based, three-piece IOL with an iris clip IOL, resulting in CMO resolution and VA of 6/9, and IOP control with single topical agent
Pathophysiology
Iris pigment dispersion, inflammation and iris neovascularisation are direct consequences of mechanical damage to the iris and ciliary body and can eventually lead to CMO¹. Elevated IOP can result from angle blockage by AC-IOL haptics, synechiae, pigment, red blood cells or inflammatory cells¹. Hyphaema, one of the less common features, results from damage to the iris and ciliary body blood vessels, which can also result in vitreous haemorrhage¹.
Presentation: key signs/symptoms
In contemporary practice, UGH syndrome should remain in the differential diagnosis for any patient who has previously undergone IOL implantation and presents with intermittent blurred vision, pain and photophobia in the corresponding eye. Other symptoms found in the literature include headaches, transient loss of vision, halos surrounding lights, ‘white outs’ and erythropsia¹.
Physical signs elicited upon examination include reduced best-corrected visual acuity (BCVA), increased IOP, hyphaema or microhyphaema, anterior uveitis (sometimes subtle), iris transillumination defects, posterior synechiae, keratic precipitates on the IOL, IOL or haptic subluxation or tilt, pseudo-phacodonesis, vitreous haemorrhage, cupping of the optic nerve head and CMO¹,⁵.
UGH syndrome can manifest weeks to years post-operatively regardless of how flawless the initial surgery was, with reports in the literature giving a mean time of 3.2 years between initial IOL implantation and UGH diagnosis⁵, with more recent studies indicating a median time of 6 or 7.5 years²,³.
Aside from the primary techniques of slit lamp microscopy and fundoscopy, two other useful methods in our diagnostic entourage are ultrasound biomicroscopy (UBM) and anterior segment optical coherence tomography (AS-OCT)¹. UBM gives a detailed overview of the anterior segment structures by adopting a high-resolution ultrasound technique that allows for non-invasive in vivo imaging⁶. It is the primary supportive modality of imaging used in the diagnosis and evaluation of UGH syndrome¹. UBM can also guide surgical management by revealing the position of the IOL in relation to other anterior segment structures such as the iris and the capsular bag¹,⁵. Anterior segment OCT also demonstrates IOL displacement and gives a greater resolution compared to UBM, and it is generally faster and less operator dependent. It’s disadvantage, however, is that it obviously cannot provide images posterior to the iris¹.
Management
The treatment of UGH syndrome differs according to the presentation. IOL explantation with or without IOL replacement is usually the traditional, definitive management solution¹. That being said, a vast proportion of cases are minimally symptomatic with many being undiagnosed, and generally UGH resolves with conservative management consisting primarily of topical steroids and non-steroidal anti-inflammatories³,⁷. These topical therapies primarily reduce inflammation and thereby CMO.
Additional topical therapies include cycloplegics, to minimise iris chaffing, and prostaglandin analogues, beta adrenergic antagonists and alpha adrenergic agonists to target increased IOP¹,⁷.
In the largest retrospective, case-control study to date of 71 UGH syndrome cases, surgical intervention was found to be superior to conservative treatment in achieving IOP reductions and BCVA improvements².
Upon deciding on surgical management for the medically refractory cases of UGH syndrome, the subsequent choices facing the surgeon are: 1) reposition the original IOL or, if explanting, 2) implant a new IOL or 3) leave the eye aphakic¹. This decision differs on a case-by-case basis.
When choosing a secondary IOL, a large review conducted by Wagoner et al concluded that complication rates, including corneal decompensation and chronic CMO, were relatively low and, perhaps surprisingly, equivocal amongst AC-IOLs, scleral-sutured IOLs or iris-sutured IOLs in those without capsular support⁸. This led the authors to conclude that no single implant or implant location resulted in superior patient outcomes compared to the others, which has also been supported by a number of other studies¹.
Nonetheless, surgical intervention does not guarantee resolution². Many patients may still require additional medical treatment after IOL exchange, such as steroids to manage uveitis or CMO, or subsequent surgery to control glaucoma¹. Even after UGH syndrome resolution following IOL exchange or surgery, over half of patients without pre-existing glaucoma still required glaucoma therapy². Other complications include recurrent UGH syndrome (Fig 4), retinal detachment, endophthalmitis and suture erosion¹,⁸.
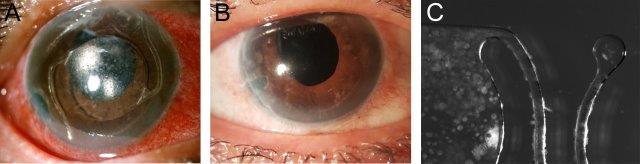
Fig 4. A case of recurrent UGH syndrome. The original AC-IOL was exchanged for a contemporary AC-IOL 10 years after the index cataract surgery. A) One year later, recurring UGH syndrome: marked circumcorneal injection, a clear cornea and relatively quiet anterior chamber (flare + with occasional cell) with a diagonally positioned angle-fixated AC-IOL, noting widespread keratic precipitates on the IOL optic and haptics. VA was 6/24. The AC-IOL was exchanged with a scleral-sutured posterior chamber IOL accompanied by an anterior vitrectomy. B) Three months post-op, the cornea was clear with the posterior-chamber IOL secured in good position. Vision was restored to 6/9.5 unaided. C) Stereo microscope image of the explanted AC-IOL under side illumination showing the distribution of widespread keratic precipitates
Conclusion
Although UGH syndrome is now relatively uncommon with phacoemulsification surgery, improved IOL design and in the bag fixation, it still occurs often, with much reduced dramatic signs and symptoms, while hyphaema may be minimal or absent. Therefore, the clinician should remain vigilant and consider this cause is the differential when patients present with photophobia, low grade anterior uveitis, recurring CMO and reduced visual acuity following cataract surgery.
Acknowledgements: We would like to thank Professor Charles McGhee for providing the authors with clinical images and illustrative cases to support this review.
References
- Le, T.; Rhee, D.; Sozeri, Y. Uveitis–Glaucoma–Hyphema Syndrome:a Review and Exploration of New Concepts. Current Ophthalmology Reports 2020, 8, 165-171
- Armonaite, L.; Behndig, A. Seventy-one cases of uveitis-glaucoma-hyphaema syndrome.Acta Ophthalmol 2020, 10.1111/aos.14477
- Elhusseiny, A.M.; Lee, R.K.; Smiddy, W.E. Surgical management of uveitis-glaucoma-hyphema syndrome.Int J Ophthalmol 2020, 13, 935-940
- Ellingson, F.T. The uveitis-glaucoma-hyphema syndrome associated with the Mark VIII anterior chamber lens implant.J Am Intraocul Implant Soc 1978, 4, 50-53
- Cheung, A.Y.; Price, J.M.; Hart, J.C., Jr. Late-onset uveitis-glaucoma-hyphema syndrome caused by Soemmering ring cataract.Canadian journal of ophthalmology. Journal canadien d'ophtalmologie 2019, 54, 445-450
- Dada, T.; Gadia, R.; Sharma, A.; Ichhpujani, P.; Bali, S.J.; Bhartiya, S.; Panda, A. Ultrasound biomicroscopy in glaucoma.Survey of ophthalmology 2011, 56, 433-450
- Shi, J.; Han, J.V.; McGhee, C.N.J.; Zhang, J. Vision threatening uveitis-glaucoma-hyphaema syndrome: An uncommon but continuing concern in the 21st century.Clinical & experimental ophthalmology 2020, 48, 127-130
- Wagoner, M.D.; Cox, T.A.; Ariyasu, R.G.; Jacobs, D.S.; Karp, C.L. Intraocular lens implantation in the absence of capsular support: a report by the American Academy of Ophthalmology.Ophthalmology 2003, 110, 840-859
Dr Jane Shi graduated from the University of Auckland and is pursuing a career in ophthalmology.
Dr Jina Han is a RANZCO registrar who graduated from Otago and completed an MD in research on cataract risk stratification at the University of Auckland.
Dr Jie Zhang is a senior HRC research fellow and scientist in the Department of Ophthalmology, University of Auckland, with interests in both laboratory and clinical research.