Syfovre side-effect blow
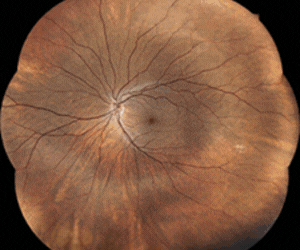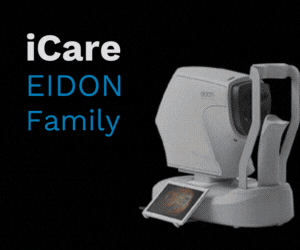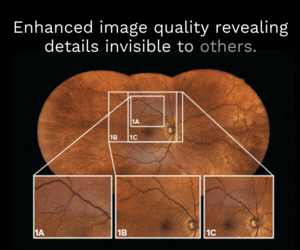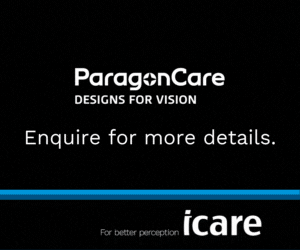
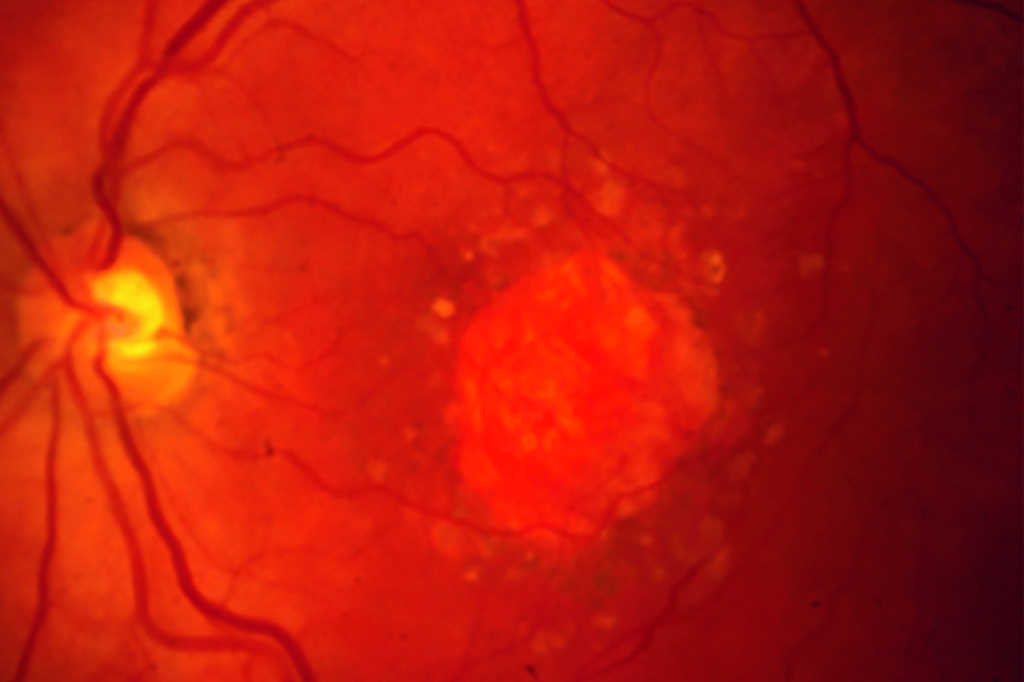
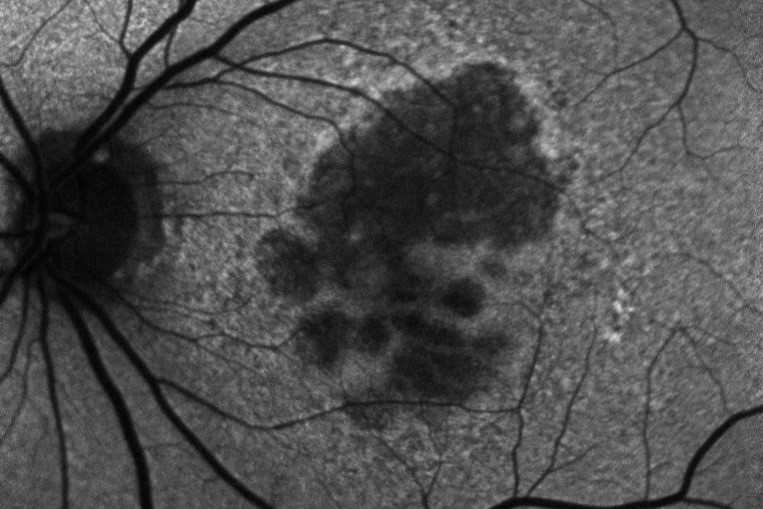
Apellis Pharmaceuticals’ Syfovre, the first FDA-approved drug for geographic atrophy, has been associated with six cases of occlusive retinal vasculitis, warned The American Society of Retinal Specialists (ASRS).
In a letter to doctors the ASRS said potentially blinding occlusive retinal vasculitis and cases of inflammation were observed one or two weeks after patients’ first Syfovre (pegcetacoplan) injection. Apellis’ share price subsequently sank by around 66% – from US$92.32 on 28 June to $31.22 on 22 August.
However, the drug's overall real-world safety profile has been consistent with its clinical trials, with occurrences of retinal vasculitis reported at a rate of approximately 0.01% per injection and no such instances flagged in the drug’s pivotal phase 3 studies, an Apellis spokesperson told Fierce Pharma.
“We are in the process of thoroughly investigating all cases with external experts, including real-world procedure technique and potential risk factors,” the spokesperson said. “Patient safety is our top priority and we take any adverse event seriously.”