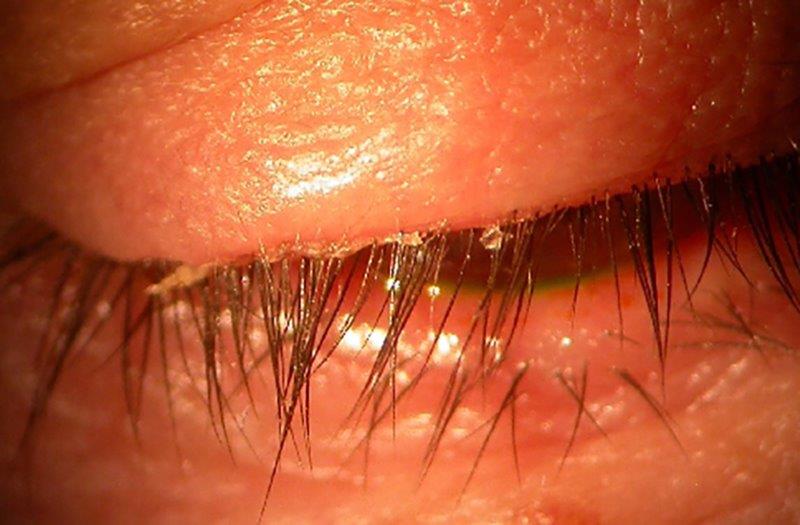
What’s new in dry eye management today and looking ahead
In the early days of my clinical career, most products for managing dry eye disease (DED) focused on increasing eye lubrication using a limited range of ingredients. Although artificial tears remain a mainstay of DED management today, there now exists a plethora of products for eyecare practitioners that can be tailored to the needs of each of our patients, including lid hygiene, ointments, punctal plugs, oral and systemic medication, omega 3 supplementation, contact lenses and in-office procedures1.
As our collective understanding of the pathophysiology of DED evolves, the pharmaceutical industry continues to deliver innovative products to address inflammation – lubricants that have complex formulations, and products for lid hygiene to decrease the overgrowth of microorganisms responsible for blepharitis. Here I focus on some newly available products and others still in the pipeline.
Managing inflammation
Inflammation is recognised as a core feature in the pathophysiology of DED2-3. Anti-inflammatories and immunomodulators, such as cyclosporine 0.05% (Restasis, Allergan), cyclosporine 0.09% (Cequa, Sun Pharmaceutical Industries), cyclosporine 0.1% (Ikervis, Santen) and lifitegrast 5% (Xiidra, Novartis), have become widely accepted in the management of DED across many parts of the world (though they are not yet commercially available in New Zealand, where patients are limited to compounded cyclosporin A).
These products are typically reserved for longer-term use (more than three months) and have a slow onset (one to four months) before the patient or the eyecare practitioners can discern a therapeutic benefit. External factors such as air conditioning, heating, wind, excessive screen time, allergens and preservatives in cosmetics can trigger additional episodic inflammatory flare-ups in DED patients. Until recently, no short-term anti-inflammatory has been approved to alleviate these flare ups.
Addressing this gap, Loteprednol etabonate is an ophthalmic corticosteroid prednisolone analogue. Upon instillation in the eye, enzymes transform it to an inactive metabolite, minimising systemic absorption. Consequently, loteprednol has lower potential than prednisolone or dexamethasone for increasing intraocular pressure. Loteprednol exists as a 0.5% ophthalmic suspension (Lotemax, Bausch+Lomb)4 for post-operative inflammation, at 0.2% (Alrex, Bausch+Lomb)5 for short-term relief of seasonal allergic conjunctivitis, and as a gel of 0.38% (Lotemax SM, Bausch+Lomb)6 for post-cataract pain and inflammation. In 2020, the Food and Drug Administration (FDA) approved a 0.25% ophthalmic suspension of loteprednol etabonate (Eysuvis, Kala Pharmaceuticals) for the short-term (up to two weeks) management of DED signs and symptoms7-8.
Multicentre, randomised, double-masked, controlled phase 2 and phase 3 trials were performed on dry eye participants to evaluate the safety profile of 0.25% loteprednol etabonate9. One group received the test product (n=1,430), while the other received a placebo (n=1,438) four times a day for two to four weeks. Loteprednol etabonate 0.25% was found to be safe and well tolerated with minimal adverse events, which included instillation site pain (5.2% of the test group) and intraocular pressure elevation (>5mmHg) with a low incidence at 0.6% in the test group. No difference was noted between the test product and placebo for visual acuity, slit lamp findings and ophthalmoscopy findings. Although not yet available in New Zealand, loteprednol etabonate 0.25% is available in several markets, including Australia, adding to the eyecare professional’s armamentarium to manage short-term flare-ups of DED.
New nasal spray
One product in the DED therapeutic pipeline was born from repurposing the smoking cessation drug, varenicline, a nicotinic acetylcholine receptor agonist. When used as a nasal spray it activates the trigeminal parasympathetic pathway and stimulates the lacrimal gland to produce more tears.
Preliminary studies were presented at the ARVO 2021 meeting on varenicline nasal spray (OC-01, Oyster Point Pharma) with DED patients randomised into three groups, using 0.6mg/ml and 1.2mg/ml varenicline and a control vehicle10. The primary endpoint of the study was an improvement in Schirmer’s test scores of >10mm at week four. The mean change of Schirmer’s test score from baseline was 11.3mm, 11.5mm and 6.3mm in the 0.6mg/ml, 1.2mg/ml and vehicle groups, respectively. Significant improvement in symptoms was noted at weeks two and four only for the test groups. The most significant adverse event among the test groups was sneezing. The advantage of this product is two-fold: the nasal route essentially eliminates treatment-associated ocular stinging/burning and the product stimulates the production of natural tears. To date, this is the only pharmaceutical nasal delivery system to be used to manage DED.
Dealing to Demodex
Blepharitis due to Demodex infestation has attracted much attention in the literature, as an overgrowth of the mites can lead to lash loss and lid and ocular surface inflammation11. Lid hygiene with tea-tree oil, its derivatives or hypochlorous acid have demonstrated efficacy at reducing the mites and the cylindrical dandruff (CD) on the lashes that are pathognomonic of the condition12-13. Despite these advancements, compliance and discomfort with lid hygiene products remain an issue (see p18).
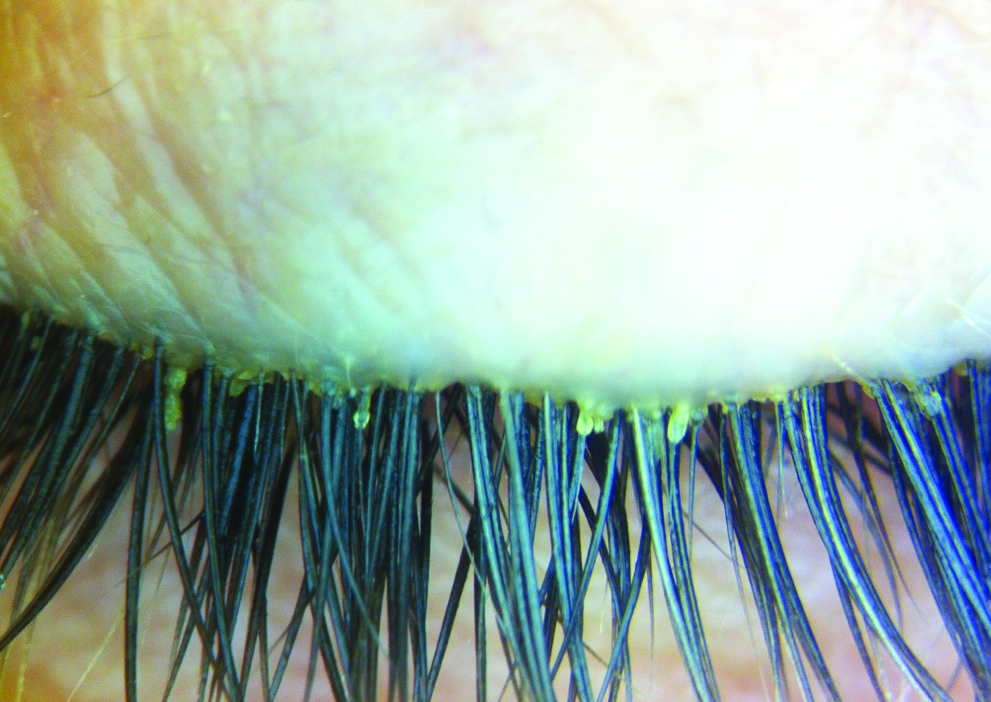
Cylindrical eyelash crusting considered pathognomonic of Demodex infestation
Recently, a pilot study investigated an ophthalmic acaricide, lotilaner ophthalmic solution 0.25% (TP-03, Tarsus Pharmaceuticals), for its safety and efficacy at decreasing Demodex infestation of the lids14. Participants with >10 CD on the upper eyelashes were enrolled to use one drop of TP-03 twice a day for 28 days. Fifteen participants were assessed at days 2, 7, 14, 28, 60 and 90. Mite density and CD grading were significantly reduced (p<0.003) at day 28 and this was sustained through to the 90-day visit. No adverse events were reported and no significant changes were noted for intraocular pressure, visual acuity or slit lamp findings.
These promising results are being evaluated further in a larger clinical study (SATURN-1)15, underway in 2021.
Conclusion
It’s an exciting time to manage dry eye, as new products come to market with many others in the pipeline, offering eyecare practitioners additional tools to help alleviate the signs and symptoms of DED.
References
- Wolffsohn JS, Travé Huarte S, Jones L et al. Clinical practice patterns in the management of dry eye disease: A TFOS international survey. Ocul Surf. 2021 May 6;21:78-86
- Bron AJ, de Paiva CS, Chauhan SK et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017 Jul;15(3):438-510
- Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Opthalmol 2012;130-90-100
- Lotemax, Bausch and Lomb. Information retrieved on July 11 2021 from https://www.bausch.ca/wp-content/uploads/2020/01/Lotemax-Monograph-Consumer-English.pdf
- Alrex, Bausch and Lomb. Information retrieved on July 11 2021 from https://www.bausch.ca/wp-content/uploads/2019/12/Alrex-PM-EN.pdf
- Lotemax SM, Bausch and Lomb. Information retrieved on July 11 2021 from https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208219s000lbl.pdf
- Eysuvis, Kala Pharmaceuticals. Information retrieved on July 11 2021 from https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/210933s000lbl.pdf
- Eysuvis, Kala Pharmaceuticals. Information retrieved on July 11 2021 from https://www.eysuvis.com/efficacy/
- Korenfeld M, Nichols KK, Goldberg D, Evans D, Sall K, Foulks G, Coultas S, Brazzell K. Safety of KPI-121 Ophthalmic Suspension 0.25% in Patients With Dry Eye Disease: A Pooled Analysis of 4 Multicenter, Randomized, Vehicle-Controlled Studies. Cornea. 2021 May 1;40(5):564-570
- Varenicline. Information retrieved on July 11, 2021 from https://www.practiceupdate.com/content/arvo-2021-varenicline-nasal-spray-provides-relief-for-dry-eye-disease-in-phase-iii-study/118091
- Bitton E, Aumond S. Demodex and eye disease: a review. Clin Exp Optom. 2021 Apr;104(3):285-294. doi: 10.1111/cxo.13123. Epub 2021 Apr 1. PMID: 32885484.
- Gao YY, Di Pascuale MA, Li W, Liu DT, Baradaran-Rafii A, Elizondo A, Kawakita T, Raju VK, Tseng SC. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005 Sep;46(9):3089-94. doi: 10.1167/iovs.05-0275. PMID: 16123406.
- Liu W, Gong L. Anti-demodectic effects of okra eyelid patch in Demodex blepharitis compared with tea tree oil. Exp Ther Med. 2021;21(4):338. doi:10.3892/etm.2021.9769
- Ouiroz-Mercado H, Ramos-Betancourt N, Cooredor-Ortega C et al. Pilot study to evaluate the efficacy of TP-03 for the treatment of blepharitis due to Demodex infestation (Mars study). IOVS 2020 June;61:2984 retrieved on July 11 2021 from https://iovs.arvojournals.org/article.aspx?articleid=2767821
- Saturn-1, Clinical trials. Information retrieved on July 11, 2021 from https://clinicaltrials.gov/ct2/show/NCT04475432?cond=demodex+blepharitis&draw=2&rank=2

Professor Etty Bitton is director of the Dry Eye Clinic at the University of Montréal, Quebec, Canada, a TFOS ambassador and she participated in TFOS DEWS II which redefined dry eye.