All go for NZ rapid Avastin extension study
A New Zealand study has been launched to establish the safety and efficacy of rapid treatment extension of Avastin (bevacizumab), the first-line anti-VEGF agent for neovascular age-related macular degeneration (nAMD), available at a fraction of the cost of aflibercept (Eylea).
The Palmerston North Interventional Rapid Avastin Treat and Extend (Pirate) study is being conducted by Drs Antoine Bonnet, Vidit Singh and John Ah-Chan, with details shared as a poster at RANZCO NZ.
Dr Ah-Chan said the project was conceived in the wake of what he called the national ophthalmology follow-up crisis of 2018, where patients in the care of the former district health boards “needlessly suffered significant visual loss and blindness due to delays of scheduled follow-ups”. However, when Covid hit in 2020 and only sight-threatening acute cases were seen and surgery performed, the team noticed not all AMD patients were receiving their scheduled injections on time. “Despite the majority of patients with wet AMD being on a standard treat-and-extend protocol with extension intervals of two weeks, we observed a number of patients whose treatment was unavoidably delayed did not suffer poorer visual outcomes,” said Dr Ah-Chan. So, with the 2020 Aries and 2021 Altair trials demonstrating patients could be extended four weeks with Eylea, we wondered if these findings could be translated to low-risk wet AMD with Avastin, he said.
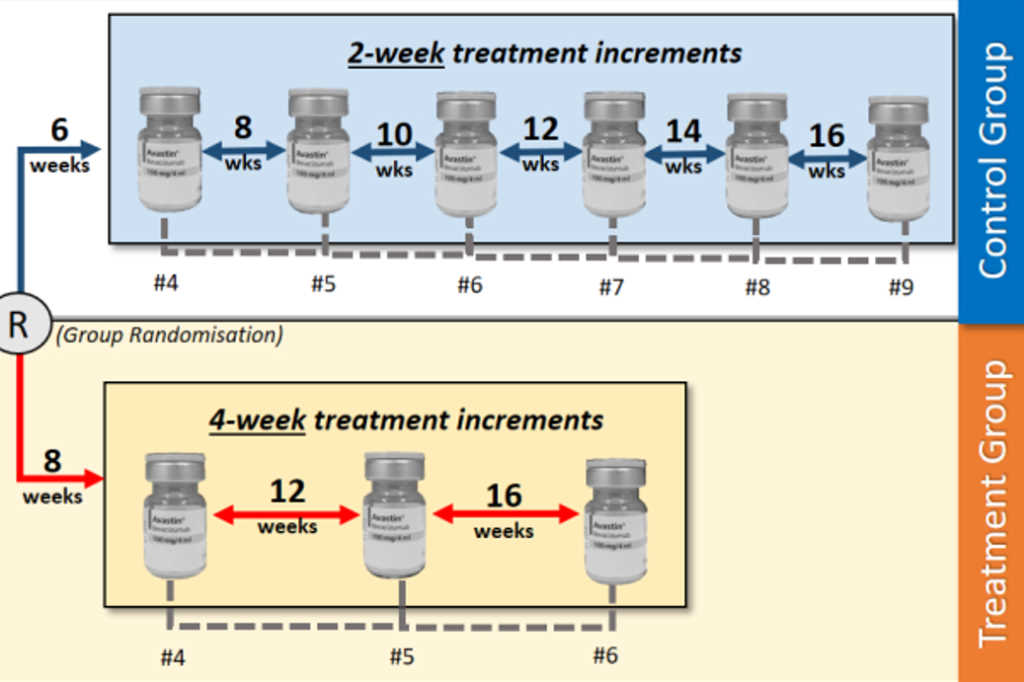
The New Zealand Pirate study includes three Avastin injections at four-week intervals. “If at four-week follow-up there’s no response, patients have Eylea (as per Pharmac requirements for approval). If the macula is dry, the patient is randomised into standard two-weekly extension or four-weekly extension to a maximum of 16 weeks, if still dry,” explained Dr Ah-Chan. “It differs from the current protocol in the number of injections and visits by patients and halves the number after induction, while maintaining visual outcomes and improving patient and caregiver experience. Obviously not all patients will get to 16 weeks.”
Applying the new regimen to Palmerston North Hospital would potentially release capacity, while maintaining visual outcomes and could be adapted to areas with similar demographics, geographics and resource constraints, he said. “We also expect a reduction in costs. In fact, our registrar Dr James Lewis (who happens to have a commerce background) is about to conduct a health economic evaluation.”
Treatment group participants are expected to require three fewer injections to reach a maximum 16-week treatment interval and will reach this target 6.5 months sooner, assuming expected treatment response, added Dr Singh. “This would result in a cost saving of $279.22 per patient in pharmaceutical consumables alone. Further savings would result from injection-related overheads, administration and labour costs,” he said.
Prospective trial recruitment will occur over an initial 12-month period with a study duration of 18-24 months. Formal recruitment is expected to commence in the coming months.