Asymmetric diabetic retinopathy
Diabetic retinopathy (DR) is one of the microvascular complications of diabetes mellitus. In most patients it is symmetrical between the two eyes. In 5-10% of patients, however, DR can be asymmetric1,2.
There is no standard definition of asymmetric DR (aDR), with various definitions being used in the literature. Gay and Rosenbaum3 defined it as a difference of at least two stages between the two eyes persisting for one year, while Duker et al defined it as the presence of high-risk proliferative DR in one eye and no proliferative or pre-proliferative DR in the other eye, persisting for two years1. The unifying features of these and other definitions are differences in the stages of retinopathy between the two eyes and its persistence over time. The progression of DR may differ between the two eyes for over one year. Hence, it is vital for the difference in DR status to persist between the two eyes for the diagnosis of aDR. Several factors contribute to the condition.
Carotid obstructive disease
Carotid obstructive disease (COD) is the most common factor responsible for aDR and has been extensively reported in the literature. The association of COD and aDR is rather complex. COD can cause worsening of DR or may have a protective effect ipsilaterally, depending upon the relative time of onset and severity of COD and DR and other vascular factors including hypertension (HTN). If significant COD develops after the start of substantial DR, it can cause a worsening of DR ipsilaterally due to added ischaemia1. However, if moderate COD develops before the onset of DR, particularly with coexistent hypertension, it has a protective effect ipsilaterally as the deleterious effects of HTN are reduced.
Ocular ischaemic syndrome
The common causes of ocular ischaemic syndrome (OIS) are atherosclerosis of the internal carotid artery or ophthalmic artery, dissecting aneurysm of the carotid artery, Takayasu arteritis and giant cell arteritis. Unilateral OIS can further worsen ischaemia of the retinal tissues, amplifying the manifestations of DR. At times, it may be difficult to differentiate the retinal manifestation of OIS from DR. The former is commonly associated with mid-peripheral blot retinal haemorrhages. The fundus fluorescein angiography (FFA) features of OIS are an increase in arm-retinal time, prolonged arteriovenous transit, choroidal perfusion deficit and late staining of arteries and veins4.
Refractive error
Axial myopia is found to be protective against proliferative DR. Degenerative changes in the retina and choroid resulting from axial length elongation cause decreased metabolic demand, which eventually helps to reduce the effects of hypoxia in diabetes and possibly decrease the production of inflammatory proangiogenic cytokines and vascular endothelial growth factor (VEGF). This has a positive impact on DR. High rates of posterior vitreous detachment in a myopic eye are also associated with a reduced rate of DR progression. Kim D et al found that axial length >25mm and choroidal thickness <250µm can have a protective effect against DR5.
Retinal or chorioretinal degenerative, dystrophic or atrophic changes
Significant loss of retinal tissue (resulting from extensive unilateral chorioretinal coloboma, unilateral retinal vascular occlusion, unilateral retinal dystrophies)6, loss of ganglion cells, or retinal nerve fibre layer (as in glaucomatous or non-glaucomatous optic atrophy)6 or posterior vitreous detachment5 can lead to aDR. The relative decrease in metabolic demand due to the loss of chorioretinal tissues mitigates ischaemia-related changes. This has a protective effect on the occurrence of DR. In addition, the thinner outer retinal tissue increases the diffusion of oxygen through different layers of the retina and prevents ischaemia.
Intraocular surgeries
Ocular surgeries such as phacoemulsification or trabeculectomy can exacerbate DR. It has been postulated this is due to the breakdown of the blood-ocular barrier, release of prostaglandins, and enhanced inflammation in diabetics7. However, a few authors have argued that uncomplicated isolated phacoemulsification has minimal impact on DR8. On the other hand, vitrectomy has been shown to have a protective effect against DR by increasing diffusion and clearance of the sequestrated ischaemic factors. Vitrectomy positively impacts the oxygenation of retinal tissue, thereby reducing hypoxia and VEGF production and improving perifoveal circulation9.
aDR diagnoses: case 1
A 58-year-old male was referred to the medical retina clinic in 2021 for the right eye (RE) (Fig 1a) severe non-proliferative diabetic retinopathy (NPDR) and no DR in the left eye (LE) (Fig 1b). He had a 20-year history of perception of light (PL+) only in the RE secondary to optic atrophy of unknown cause. A non-contrast MRI of the brain and orbits performed 20 years ago was reported to be normal. LE vision at presentation was 6/6. Medical history included diabetes mellitus (DM) (HbA1c 64mmol/mol), hypertension and dyslipidemia. Initially, he has referred in 2017 with RE moderate NPDR and LE with no DR. Lacking follow-up, he was re-referred in 2021. Clinically, the optic nerve function in the LE was normal.
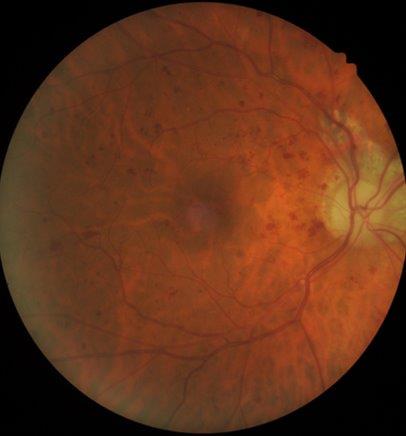
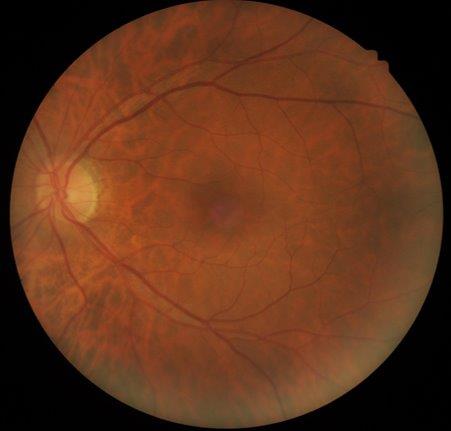
Fig 1a. Case 1’s RE at presentation (left) and Fig 1b. Case 1’s LE at presentation (right)
aDR diagnoses: case 2
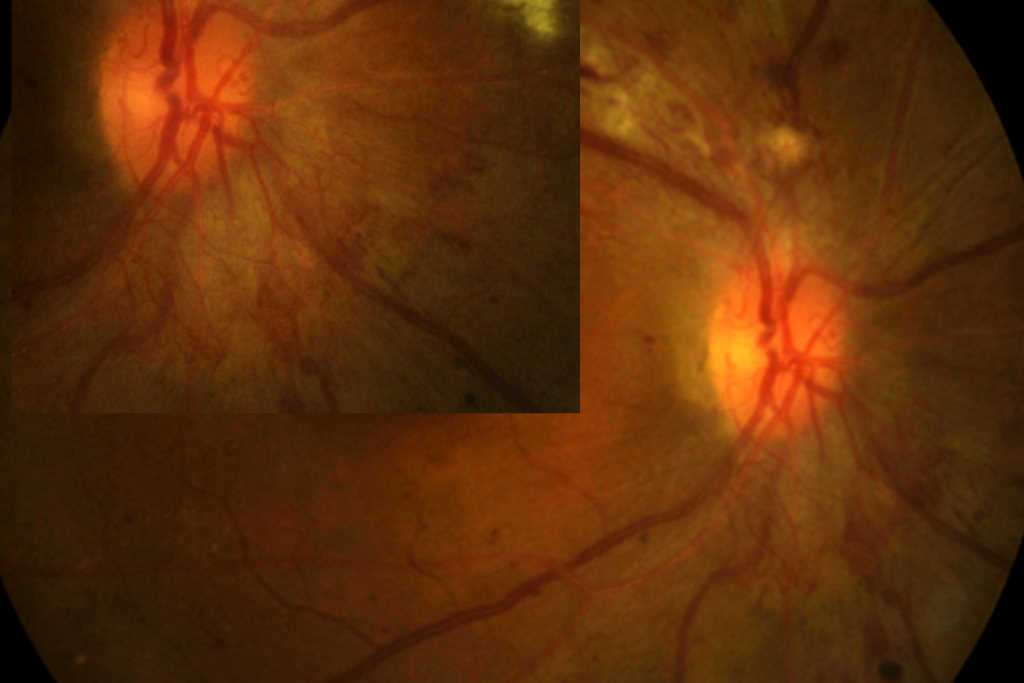
A 48-year-old female was referred to the neuro-ophthalmology clinic for a decrease in vision in the RE over the preceding 4-6 months. Her medical history included DM (HbA1c 98mmol/mol at presentation), HTN, dyslipidemia and diabetic nephropathy. She had missed her scheduled appointments for diabetic photo screening. She was noted to have visual acuity of 6/36 in the RE and 6/12 in the LE. Ocular examination revealed 6+ posterior subcapsular (PSC) cataract in the RE and 3+ PSC in the LE. Retinal examination showed RE high-risk proliferative diabetic retinopathy with neovascularisation of the optic disc and optic disc oedema (Fig 2a) while LE showed partial optic atrophy with mild NPDR (Fig 2b).
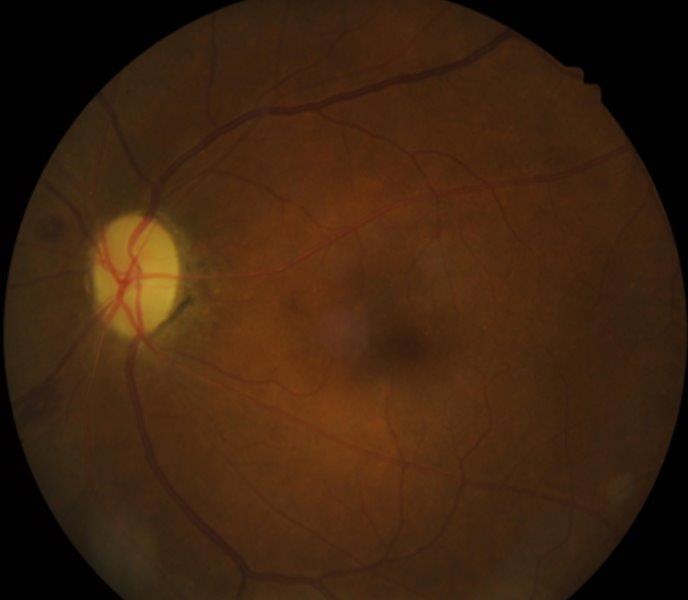
Fig 2b. Case 2’s LE at presentation
Management
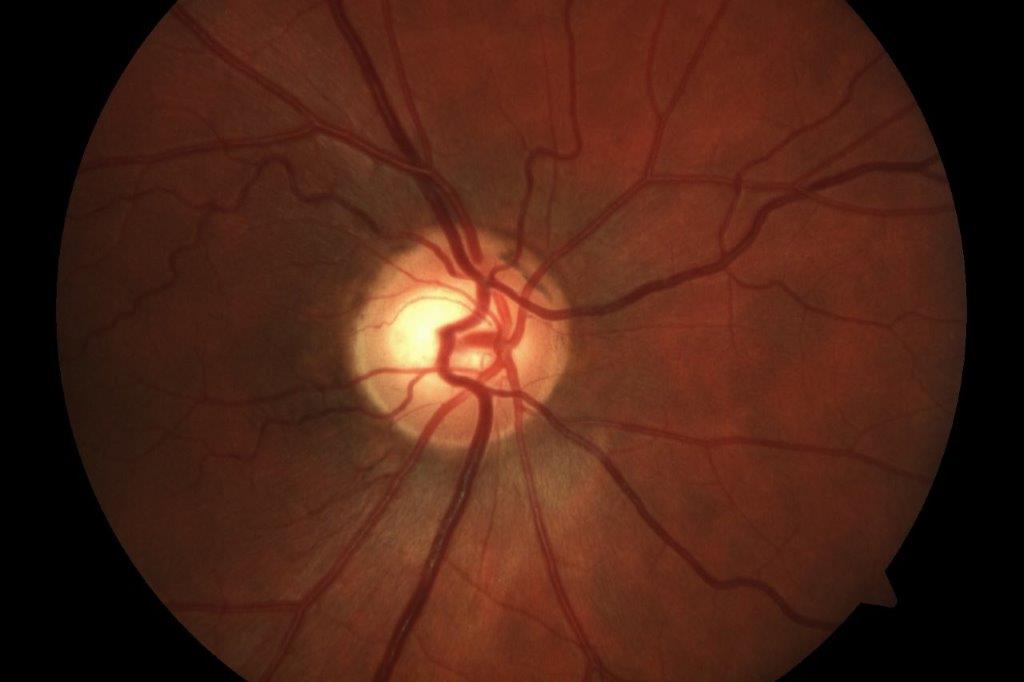
In case 1, a carotid doppler did not reveal any significant carotid stenosis. LE visual field assessment was normal. MRI of the brain and orbits showed RE sphenoid meningioma with extension, along with optic nerve sheath stopping short of the posterior sclera (Fig 1c). He underwent surgical debulking and the RE retinal photo four weeks post-debulking showed a reduction in retinal haemorrhages (Fig 1d). Hence, this was not a case of aDR; rather, the retinal haemorrhages were secondary to mechanical compression of the optic nerve and central retinal vein.
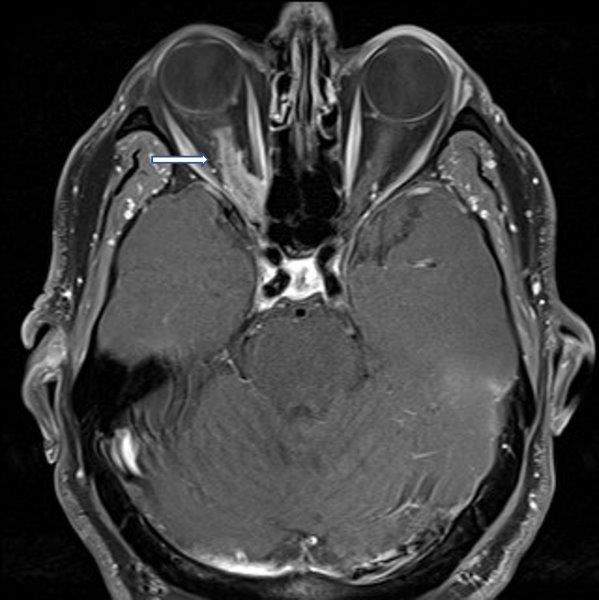
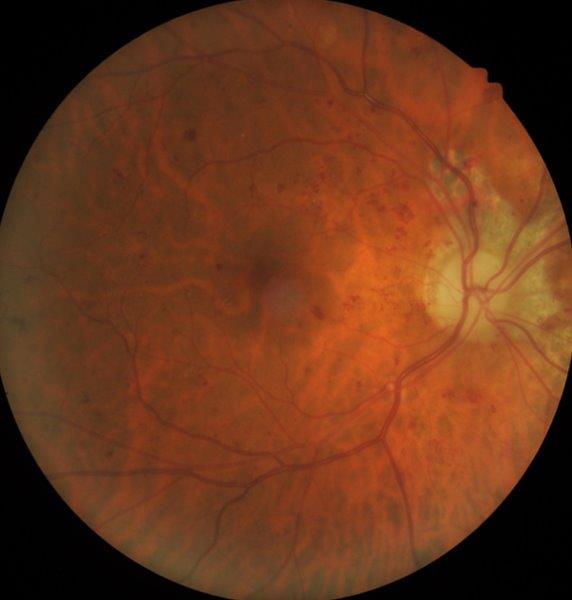
Fig 1c. Case 1’s MRI demonstrating (arrow) meningioma along the optic nerve sheath
stopping short of posterior sclera (left). Fig 1d. Case 1’s RE retinal haemorrhages, a
month after debulking of meningioma (right)
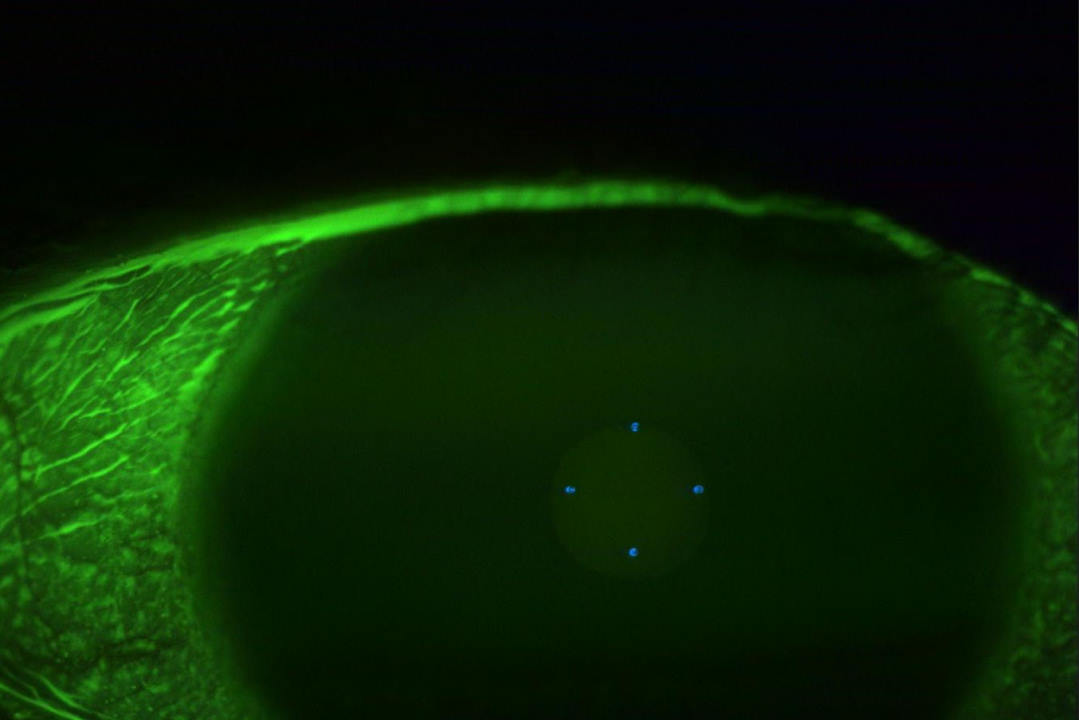
Case 2 underwent urgent neuroimaging through the emergency department. An MRI of the brain and orbit was normal. Carotid doppler did not reveal any significant carotid stenosis. FFA did not suggest features of ocular ischaemia. She was started on a four-weekly schedule of anti-VEGF and listed for pan-retinal photocoagulation. The work-up for LE optic neuropathy returned normal. The OCT scan of RE at presentation is shown in Fig 2c and after six months in Fig 2d.
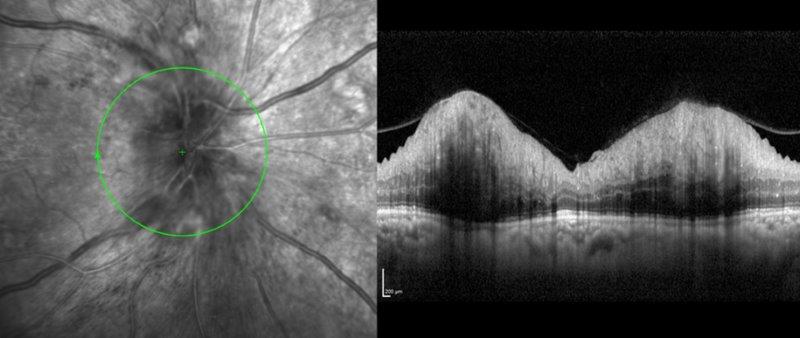
Fig 2c. Case 2’s OCT RNFL at presentation
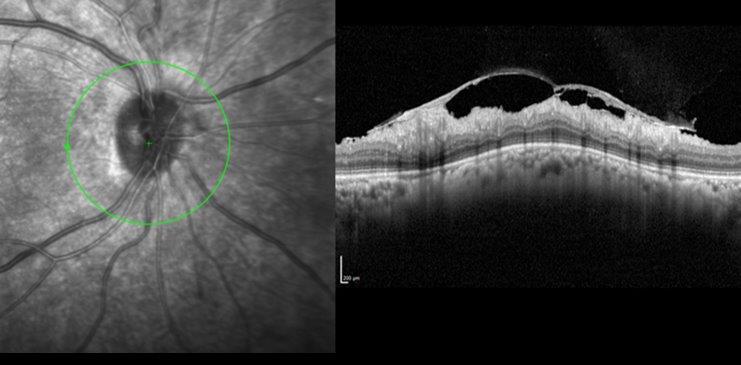
Fig 2d. Case 2’s OCT RNFL after six months of treatment
The second patient demonstrated aDR. The presence of partial optic atrophy in the LE was protective against severe DR. The first patient had optic atrophy, which is protective against DR, and his retinal haemorrhages were secondary to a different systemic condition. Hence, it is vital to understand the factors that may lead to aDR and investigate to rule out any underlying systemic disease that may present with changes similar to DR.
References
1. Duker J, Brown G, Bosley T, Colt C, Reber R. Asymmetric proliferative diabetic retinopathy and carotid artery disease. Ophthalmology 1990;97:869–74.
2. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843.
3. Gay A, Rosenbaum A. Retinal artery pressure in asymmetric diabetic retinopathy. Arch Ophthalmol 1966;75:758–62.
4. Wang H, Wang Y, Li H. Multimodality imaging assessment of ocular ischemic syndrome. J Ophthalmol 2017;2017:4169135.
5. Kim D, Song J, Kim Y, Lee J, Kim J, Yoon Y, Joe S. Asymmetric diabetic retinopathy progression in patients with axial anisometropia. Retina 2018 38: 1809-1815.
6. Dogru M, Inoue M, Nakamura M, Yamamoto M. Modifying factors related to asymmetric diabetic retinopathy. Eye 1998;12:929–33.
7. Mittra R, Borrillo J, Dev S, Mieler W, Koenig S. Retinopathy progression and visual outcomes after phacoemulsification in patients with diabetes mellitus. Arch Ophthalmol 2000;118:912–7.
8. Romero‑Aroca P, Fernández‑Ballart J, Almena‑Garcia M, Méndez‑Marín I, Salvat‑Serra M, Buil‑Calvo J. Nonproliferative diabetic retinopathy and macular oedema progression after phacoemulsification: Prospective study. J Cataract Refract Surg 2006;32:1438–44.
9. Luliano L, Corbelli E, Bandello F, Codenotti M. Protective effect of vitrectomy on the course of diabetic retinopathy: A case report Eur J Ophthal. 2022; 32:NP177–NP180.

Dr Arvind Gupta is a consultant ophthalmologist specialising in cataract, medical retina and neuro-ophthalmology. He is based at Manukau Super Clinic, Greenlane Clinical Centre and Eye Doctors in Auckland.