FDA fast-tracks Australian bionic eye
An Australian-developed bionic eye for retinitis pigmentosa (RP) patients has been granted ‘breakthrough’ designation by the US Food and Drug Administration (FDA), fast tracking its review process.
“This life-changing bionic eye can now be brought more quickly to the people who need it the most,” said Dr Ash Attia, CEO of Bionic Vision Technologies (BVT) which developed the bionic eye in partnership with the Centre for Eye Research Australia, and the Bionics Institute, Commonwealth Scientific and Industrial Research Organisation’s Data61 and Melbourne University.
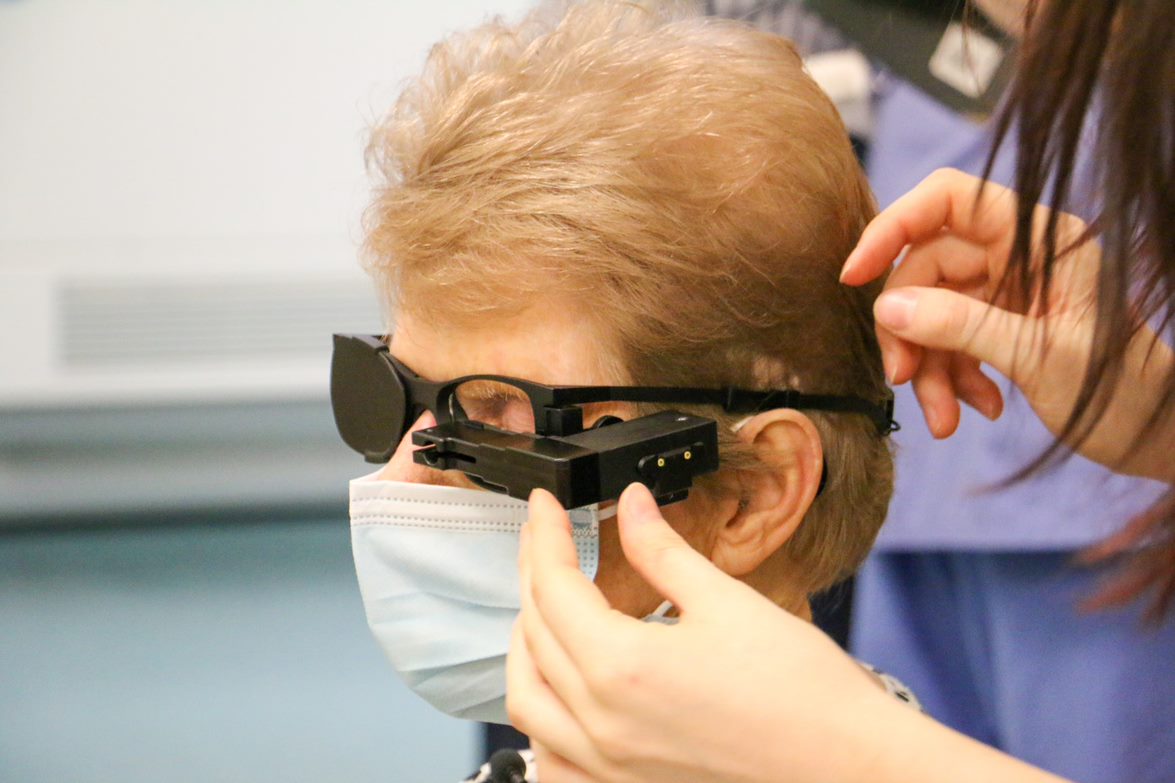
The eye consists of glasses-mounted cameras that send electrical signals to a suprachoroidal visual implant. According to a two-year feasibility study published in June 2021’s Journal of Neural Engineering, four late-stage RP patients using the device were able to identify features including traffic lights, cars, people, trees, boats and street poles. BVT said it expects to complete clinical trials and make the device available in 2024.
For more, see www.eyeonoptics.co.nz/articles/archive/bionic-eyes-no-longer-science-fiction and www.eyeonoptics.co.nz/articles/archive/bionic-eye-looks-to-human-trials