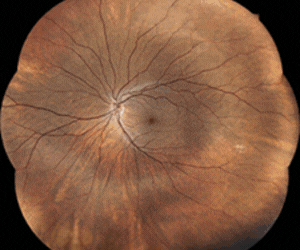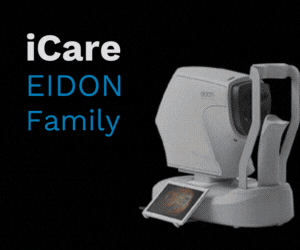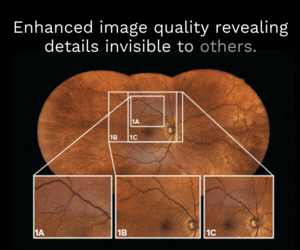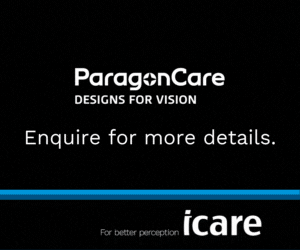
Glaukos’ milestones announced
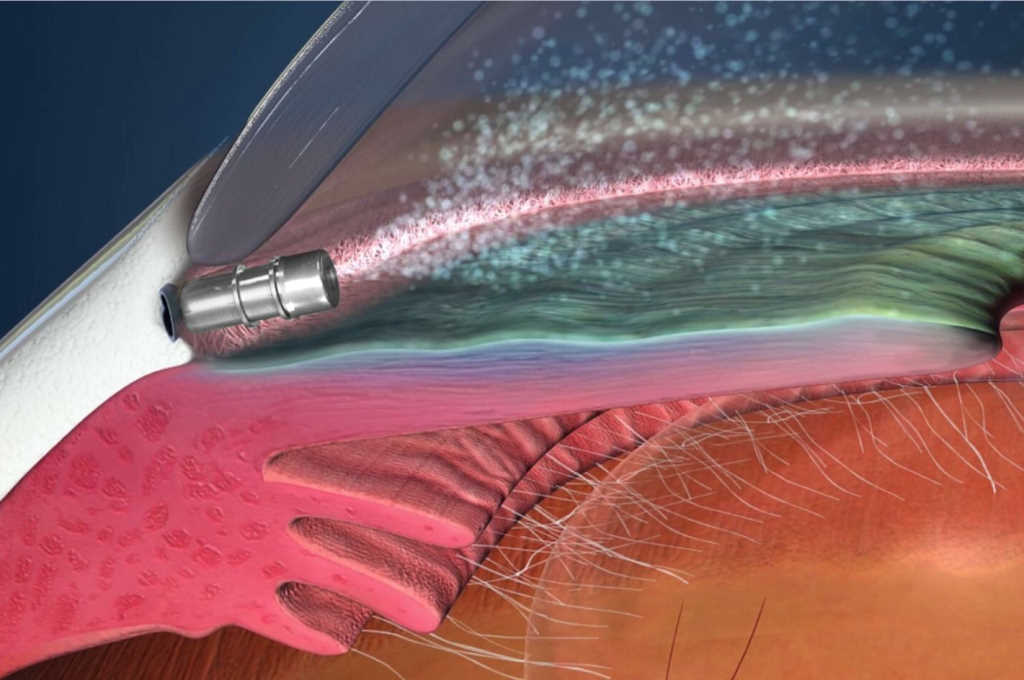
Micro-invasive glaucoma surgery pioneer Glaukos announced its iStent family of technologies have now been implanted in more than one million procedures worldwide.
“One million iStents implanted is a tremendous accomplishment,” said Professor Ike Ahmed from the John A. Moran Eye Center, Utah, US. “As one of the early adopters, I have experienced first-hand how iStent, and subsequent generations, have fundamentally revolutionised the way we think and treat glaucoma over the last decade. It’s been an incredible journey thus far and I’m excited to see what the future holds for our glaucoma patients.”
iDose TR’s positive results
In other news, Glaukos announced positive topline data for both of its phase 3 trials for iDose TR, which contains a novel formulation of travoprost, a prostaglandin analogue used to reduce intraocular pressure. Once all the travoprost is released, the iDose TR is designed to be removed and replaced with a new iDose TR, thus potentially offering an alternative to daily eye drop treatment.
The iDose TR phase 3 trial programme consists of two prospective, randomised, double-masked pivotal clinical trials designed to compare the safety and efficacy of a single administration of one of two iDose TR models with fast- and slow-release rates. Initial data showed both successfully achieved pre-specified primary efficacy endpoints through three months and demonstrated “excellent” tolerability and a favourable safety profile through 12 months, said the company.
“We are very pleased to announce these robust and replicative positive phase 3 pivotal data results, which… powerfully reaffirms our view that iDose TR can be a transformative novel technology,” said Thomas Burns, Glaukos chairman and chief executive officer.
Investing more than 30% of its revenue back into R&D, the company has a significant number of clinical studies for both current and future products ongoing, with additional studies commencing in the near future, he said.