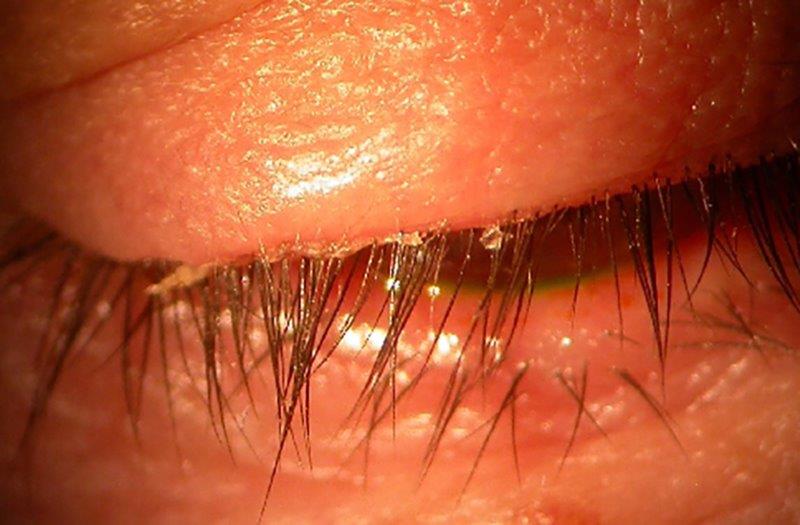
Keratolytic drug for MGD
Meibomian gland dysfunction (MGD) is characterised by alterations in glandular secretion and terminal duct obstruction, secondary to hyperkeratinisation of the ductal epithelium1. The oils secreted from the glands contain a mixture of lipid secretions and keratinised epithelial debris. Keratin levels are increased in MGD, which is understood to alter meibum consistency due to disulfide bond crosslinking of proteins arising from the acinar cells2,3. Recent in vitro studies have further demonstrated lipid film disruption due to keratin4. Such changes in meibum have the potential to promote the more prevalent evaporative form of dry eye disease (DED)5 and its associated ocular irritation symptoms.
As yet, no approved pharmacological therapies for MGD exist, so it’s exciting for the Ocular Surface Laboratory, along with a number of other research sites across Australia and New Zealand, to be involved in a large international multicentre clinical trial investigating the novel keratolytic, keratostatic and lipogenic compound, AZR-MD-001. The ointment contains selenium disulfide (SeS2; Azura Ophthalmics VIC, Australia*) and is a potential treatment for MGD.
To date, only preliminary outcomes have been presented at the 2021 Association for Vision in Research in Ophthalmology (ARVO) virtual conference. Associate Professor Laura Downie described dose-dependent improvements in dry eye symptoms and clinical meibomian gland signs at three months, relative to control. The OSL’s poster on the outcomes of a parallel multicentre study, where 0.5% AZR-MD-001 was tested for three months in patients of varying degrees of DED severity, suggested that favourable outcomes were most predictable in participants with mild to moderate DED. In another presentation from UNSW, in Sydney, Professor Fiona Stapleton’s poster described an exploratory study involving symptomatic contact lens wearers. With twice-weekly application of the AZR-MD-001 ointment, meibomian gland function improved and symptoms were reduced. Importantly, no major safety concerns were identified in any of these trials.
Collectively, the early study findings suggest that the keratolytic ointment (AZR-MD-001) has the potential to become the first effective drug treatment specifically for meibomian gland dysfunction.
Active recruitment for the multicentre trial in patients with mild-moderate DED is ongoing and patients with MGD who are interested in participating are encouraged to contact Karien Nel via k.nel@auckland.ac.nz or by calling 022 EYE PAIN (022 393 7246).
* https://eyeonoptics.co.nz/articles/archive/new-mgd-treatment-holds-promise/
References
- Nichols KK, Foulks GN, Bron AJ, et al. The International Workshop on Meibomian Gland Dysfunction: Executive Summary. Invest Ophthalmol Vis Sci. 2011;52:1922-1929.
- Tomlinson A, Bron AJ, Korb DR, et al. The International Workshop on Meibomian Gland Dysfunction: Report of the Diagnosis Subcommittee. Invest Ophthalmol Vis Sci. 2011;52:2006-2049.
- Ong BL, Hodson SA, Wigham T, et al. Evidence for Keratin proteins in normal and abnormal human meibomian fluids. Curr Eye Res. 1991; 10, 1113-1119.
- Jeyalatha MV, Qu Y, Liu Z, et al. Function of meibomian gland: Contribution of proteins. 2017; Exp Eye Res. 163:29-36.
- Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012; 31; 472-478.

Professor Jennifer Craig heads the Ocular Surface Laboratory in the Department of Ophthalmology at the University of Auckland, was vice-chair of TFOS DEWS II and is chair of the new TFOS workshop, ‘A Lifestyle Epidemic – Ocular Surface Disease’.