Real-world clinical practice on dry eye
The Save Sight Dry Eye Registry (SSDER) was established by chief investigator Professor Stephanie Watson, head of the Corneal Research Group at the Save Sight Institute, University of Sydney, in 2020. The SSDER is part of the Fight Corneal Blindness! (FCB!) project, an observational study of outcomes in patients with corneal disease1 which has tracked the outcomes of macular disease treatment in routine clinical practice. Such clinical registries in ophthalmology have existed for decades and are valuable tools for monitoring the natural history, treatment.
The SSDER was established to provide real-world data on dry eye natural history and treatment outcomes, since evidence provided by randomised controlled trials has focused on treatments with patients enrolled using strict inclusion and exclusion criteria, typically conducted over the short term. With optometrists managing many dry eye patients, there was a need to collaboratively collect data from both ophthalmology and optometry practices.
Module milestones
The FCB! project's SSDER aims to:
- Utilise world-first technology, the Save Sight Registries, to efficiently capture treatment outcomes data in real-world practice, including patient-reported outcomes to inform on the outcomes of new therapies for dry eye disease
- Inform clinical benchmarking and guideline development using real-world evidence to improve patient outcomes
- Train students in research to enable them to provide future solutions to reduce the growing burden from corneal disease, including dry eye
Module design
The SSDER is unique as it is the first collaborative registry to collect data from everyday ophthalmology and optometry clinical practice on dry eye treatment outcomes over the long-term. Dry eye diagnostic tests and clinical signs collected within the SSDER are closely based on the recommendations of the TFOS DEWS II Diagnostic Methodology report and the ‘activity’ and ‘damage’ parameters of the Ocular Surface Disease Scoring System (OSDISS)6,7. Quality assurance measures are used to ensure that only verified and high-quality data are entered into the system. Patient-reported outcomes are also collected by the registry using the Ocular Surface Disease Index (OSDI), Ocular Comfort Index (OCI) and Primary Health Questionnaire-4 (PHQ-4) to enable understanding of the impact of dry eye on quality of life and the benefit to patients of treatments in the real world.
A wide range of therapeutic interventions used in the management of patients with dry eye disease (DED) in routine clinical practice can also be recorded in the SSDER as medical treatments (eg. artificial tears, anti-inflammatories, antihistamines, lid hygiene, warm compresses, autologous serum or alternative therapy), mechanical treatments (eg. punctal plugs), physical treatments (eg. intense pulse light, lid debridement) and surgical treatments (eg. thermal cauterisation, or punctal occlusion with a conjunctival flap or graft).
The ability to collect longitudinal data will improve our understanding of dry eye natural history3, which is currently poorly understood.
Benefits
The SSDER is a free, efficient, web-based and user-friendly data collection module designed to provide maximum data security and anonymity and is compatible with electronic data entry from routine clinical practice. Its software allows clinicians to self-audit their practice with instantaneous outcome reports and graphical tracking of ocular events, procedures and treatment outcomes for patients’ visits over time for each eye (Fig 1). Using the system, individual physicians can review their data and compare them to benchmark data from the registry, participate in national and international audits, receive invitations to contribute data and co-author publications and publish their own data. The SSDER also has accreditation with the Royal Australian and New Zealand College of Ophthalmology and Optometry Australia so users can claim Continuing Professional Development points or hours, as applicable. Users in other countries also have the potential to use analysed registry data for educational accreditation.
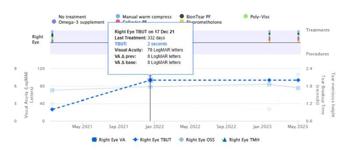
Fig 1. Example of graphical tracking of ocular treatment and procedures over time
Achievements
Since 2022, the global data from more than 1,049 eyes and 2,295 practice visits entered into the SSDER have generated a significant record of research outputs, leading to 24 influential conference presentations, 11 publications (five journal articles and six abstracts), annual ‘DryEyeClub: Seeing Outcome’ webinars and the development of promotional materials, including The View newsletters and brochures. These contributions have substantially advanced dry eye knowledge and informed clinicians in their everyday practice across Australia and internationally.
The Save Sight Dry Eye Registry has an international steering committee, with Prof Watson as chair. Members include Drs Alberto Recchioni, David Mingo, Fanny Babeau, Himal Kandel, Francisco Arnalich-Montiel and myself, Scientia Professor Fiona Stapleton and Professors Gerd Geerling, Jennifer Craig, Laura Downie, Mark Gillies, Saaeha Rauz and Vincent Daien.
FCB! Save Sight Registries access
To join or request access to the registry, visit https://savesightregistries.org/fight-corneal-blindness/. To find out more please contact us at ssi.ssr@sydney.edu.au
The Save Sight Dry Eye Registry is supported by an unrestricted educational grant from Novartis and Seqirus.
References
1. Watson S, Aguas M, Downie L, et al. The Save Sight Dry Eye Registry: One year capture of real-world data for dry eye. IOVS. 2022;63(7):1520-A0245.
2. Gillies M, Walton R, Liong J, et al. Efficient capture of high-quality data on outcomes of treatment for macular diseases: the fight retinal blindness! Project. Retina. 2014;34(1):188-195.
3. Khoo P, Downie L, Stapleton F, et al. Clinical registries in dry eye disease: a systematic review. Cornea. 2022;41(12):1572-1583.
4. Kandel H, Gillies M, Watson S. Opportunities and challenges for clinical registries. Clin Exp Optom. 2023;51(6):651-652.
5. Tan J, Ferdi A, Gillies M, Watson S. Clinical registries in ophthalmology. Ophthalmology. 2019;126(5):655-662.
6. Mathewson P, Williams G, Watson S, et al. Defining ocular surface disease activity and damage indices by an international Delphi consultation. Ocul Surf. 2017;15(1):97-111.
7. Wolffsohn J, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539-574.

Dr Ngozi Chidi-Egboka is a post-doctoral researcher and clinical trial sub-investigator for the University of Sydney’s Corneal Research Group at Save Sight Institute. Her clinical practice, teaching, research, community service and leadership activities centre around improving the delivery of anterior eye health clinical care, with a key interest in ocular surface and dry eye disease.