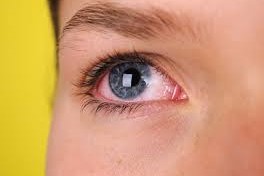
Reproxalap’s DED mission
Aldeyra has announced the submission of a New Drug Application (NDA) to the US Food and Drug Administration (FDA) for topical ocular reproxalap, an investigational new drug candidate for the treatment of signs and symptoms of dry eye disease (DED).
Reproxalap is already in late-stage development for allergic conjunctivitis, commonly associated with DED, with results of the phase-3 Invigorate-2 trial expected in 2023. The NDA submission is supported by safety and efficacy data from five adequate and well-controlled clinical trials including more than 2,000 patients, with no observed clinically significant safety concerns, according to an Aldeyra statement.
“The NDA submission for reproxalap is, to our knowledge, the most comprehensive regulatory package ever for a dry eye disease drug candidate,” said Dr Todd Brady, Aldeyra president and CEO. “With data suggesting activity within minutes of administration, reproxalap could provide an important treatment option for the millions of dry eye patients who generally regard currently available therapies as inadequate.”
Reproxalap’s mechanism is modulation of reactive aldehyde species (RASP), which are elevated in ocular and systemic inflammatory disease. If approved, it will be the first marketed RASP modulator.
For more, see www.nzoptics.co.nz/articles/archive/reproxalap-meets-primary-endpoints