Understanding and treating corneal nerve dysfunction
Dry eye disease (DED) is a multifactorial ocular surface condition that affects millions worldwide and is characterised by loss of homeostasis of the tear film, epithelial damage, inflammation and neurosensorial abnormalities1.
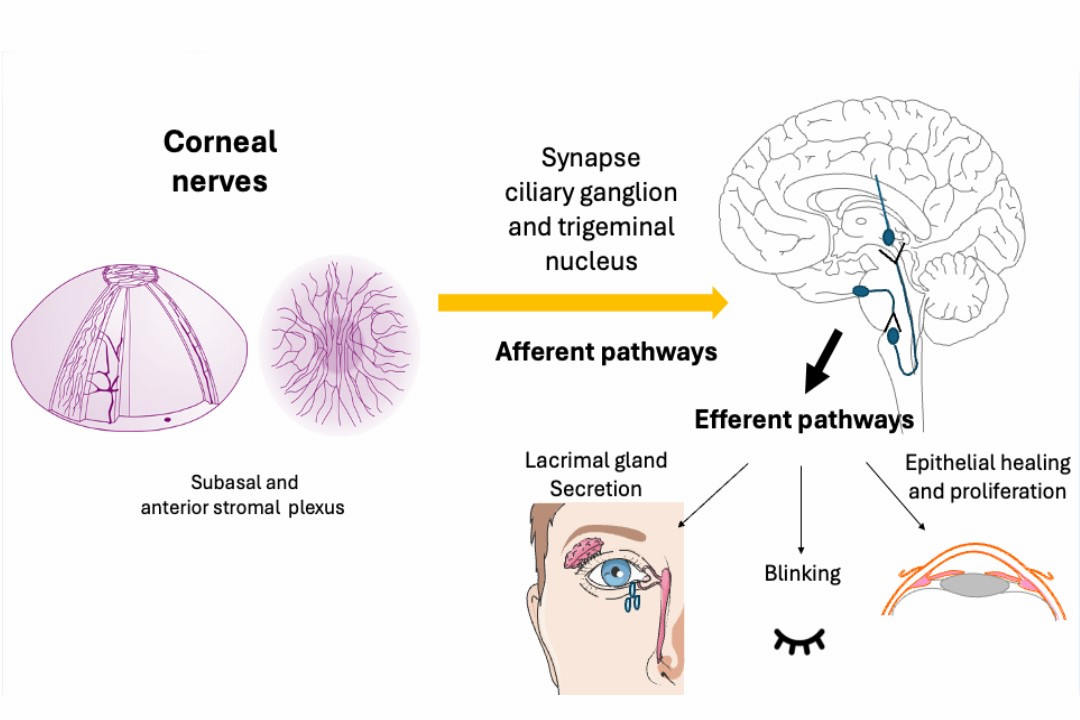
The ocular surface is densely innervated by sensory nerves, derived primarily from the ophthalmic branch of the trigeminal nerve. These complex and integrative pathways play a crucial role in regulating tear secretion, blinking reflex and epithelial renovation as shown in Fig 1. Corneal nerves serve as intrinsic coordinators of multiple mechanisms that continually maintain ocular surface integrity and turnover2. In DED, corneal nerves may be the site of neuroplastic change and sensitisation, which may explain reported symptoms such as ocular pain, burning or foreign-body sensation. In recent years, a number of potential mechanisms have been described2-4.
- Neuroinflammation: chronic inflammation of the ocular surface, a hallmark of DED pathophysiology, induces activation of nociceptive sensory nerves. This neuroinflammatory response leads to sensitisation of transient receptor potential (TRP) channels in the cornea, activating nociceptive signaling and pain perception
- Neurotrophic factor imbalance: in response to ocular stress, corneal epithelial cells release neurotrophic factors, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). NGF is a key neurotrophin involved in the development, maintenance and repair of sensory corneal nerves while BDNF is another neurotrophic factor that supports the survival and growth of sensory nerves. While initially protective and essential for maintaining corneal health and appropriate sensory nerve function, the sustained elevation of these factors can lead to hyperinnervation and abnormal sensory nerve function. The hyperinnervation may lead to symptoms such as chronic pain or discomfort, as well as impaired corneal healing.
- Corneal nerve morphology: confocal microscopy studies have demonstrated reduced corneal nerve density and altered nerve morphology in DED patients. These structural changes correlate with disease severity and chronicity
The association between pain and DED is not totally understood. A comprehensive examination encompassing biomicroscopy and ancilliary tests, such as Schirmer test, tear film osmolarity and tear breakup time, might offer diagnostic value. However, no objective assessment of corneal nerves is possible. Nerve abnormalities can be observed through in vivo confocal microscopy (IVCM), which allows real-time visualisation at a cellular level. Corneal sensitivity can be assessed using esthesiometers and cotton wisp tests.
Fig 2 demonstrates some examples of IVCM in normal eyes and those with DED. Questionnaires can be used to quantify DED patients’ frequency and severity of symptoms. To more specifically evaluate corneal pain, some specific tools have been developed, such as the Ocular Pain Assessment Survey (OPAS), that can help guide patients and physicians through the diagnosis, treatment and improvement of pain symptoms5.
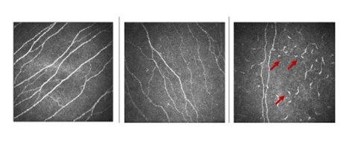
Fig 2. In vivo confocal microscopy in normal (left), in DED showing decreased nerve density (centre) and in neurotrophic keratitis showing inflammatory cells (arrow) with a decrease of nerve density (right)
It is challenging to treat corneal neuralgia and severe DED because it involves local and systemic neuronal interactions. Current strategies include control of inflammation, tear restoration, nerve regeneration and suppression of pain mechanisms, which must be added according to clinical presentation and response to therapeutic approaches, from the simpler ones first to more complex options6.
Novel therapeutic strategies targeting neurosensory pathways have arisen from understanding DED nerve dysfunction. These approaches aim to promote nerve regeneration and re-establish critical neural connections for tear production and epithelial cell maintenance:
- Neuroprotection: therapies aimed at preserving corneal nerve integrity and function are under investigation. Neurotrophic agents like NGF inhibitors and BDNF modulators seek to prevent nerve degeneration while maintaining ocular surface health
- Neuromodulation: topical medications targeting TRP channels or neurokinin receptors offer promise in alleviating neuropathic pain associated with DED
- Regenerative medicine: stem-cell therapies and growth-factor supplementation hold potential for restoring corneal nerve density and improving sensory function in severe DED cases
Thus, the association between nerve damage and DED signs and symptoms is increasingly recognised in clinical research. Nerve dysfunction in DED contributes significantly to the pathophysiology and the manifestation of clinical presentations. This can range from neurotrophic conditions with diminished tear production, compromised epithelial integrity and loss of sensitivity, to the experience of heightened ocular discomfort, characterised by sensations of burning, stinging or foreign body intrusion, indicative of nerve sensitisation and aberrant nociceptive signaling. Understanding these associations underscores the importance of targeting neurosensory pathways in the management and treatment of this complex ocular condition.
References
1. Craig JP, Nichols KK, Akpek EK et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276-283.
2. Belmonte C, Nichols JJ, Cox SM, et al. TFOS DEWS II pain and sensation report. Ocul Surf. 2017;15(3):404–437.
3. Galor A. Painful Dry Eye Symptoms: A Nerve Problem or a Tear Problem? Ophthalmology. 2019;126(5):648–651.
4. Hamrah P, Qazi Y, Shahatit BM, et al. Corneal nerve and epithelial cell alterations in corneal allodynia: an in vivo confocal microscopy case series. Ocul Surf. 2017;15(1):139–151.
5. Qazi Y, Hurwitz S, Khan S et al. Validity and Reliability of a Novel Ocular Pain Assessment Survey (OPAS) in Quantifying and Monitoring Corneal and Ocular Surface Pain. Ophthalmology.2016;123(7):1458–1468.
6. Dieckmann G, Goyal S, Hamrah P. Neuropathic Corneal Pain: Approaches for Management. Ophthalmology. 2017 Nov 1;124(11):S34–47.

Isabela Yang is a research fellow in the Department of Ophthalmology at the Tufts Medical Center, Tufts University School of Medicine, in Boston, Massachusetts, USA.

Professor Pedram Hamrah, from the Department of Ophthalmology at Tufts Medical Center, has a special interest in corneal innervation and immunology.

Associate Professor Monica Alves, from the Department of Ophthalmology and Otorhinolaryngology at the University of Campinas, Sao Paulo, Brazil, has a special interest in dry eye disease and was vice-chair of the TFOS Lifestyle Workshop.