Drug-eluting CL shows promise for glaucoma
Mediprint Ophthalmics announced the successful completion of its first study using a drug-eluting contact lens (CL) to treat mild to moderate glaucoma and ocular hypertension.
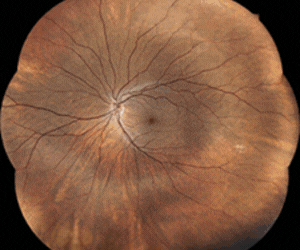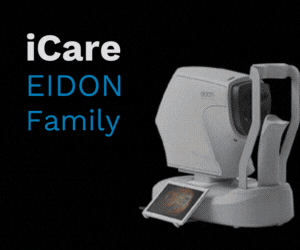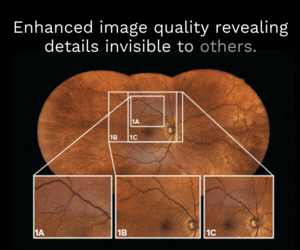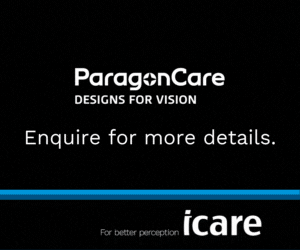
The company’s sustained innovative glaucoma and ocular hypertension treatment (SIGHT) programme evaluated its bimatoprost-printed LLT-BMT1 contact lens in human eyes for the first time. This initial trial showed a favourable safety and tolerability profile and demonstrated efficacy from a single dose.
The SIGHT-1 study’s five subjects, whose average age was 77.4 years old and who had not previously worn contact lenses, wore a LLT-BMT1 lens in each eye for seven days continuously. Notably, the incidence of hyperaemia detected in SIGHT-1 was lower than that observed for bimatoprost drops.
“I was excited to study this investigational treatment and believe that LLT-BMT1’s unique product presentation and potential benefits differentiate it from other glaucoma treatments currently on the market,” said Dr David Wirta, Eye Research Foundations’ medical director and SIGHT-1’s principal investigator.
Mediprint will now move forward with SIGHT-2, a larger phase 2b clinical study intended to optimise dosage for efficacy, which will be followed by an even broader phase 3 study.