Genetic advances and impact on paediatric eyecare: focus on congenital cataract
Congenital cataract typically presents in infancy with variable lens opacities that block the normal red reflex (Fig 1). It can be associated with other ocular and systemic abnormalities. There may be a family history of cataract and the inherited forms are typically autosomal dominant. Diagnosing the cause of the cataract benefits the patient in understanding risk to other family members as well as risk of systemic associations. Our approach to the investigation of these patients has evolved to include next-generation sequencing (NGS) gene panel investigation.
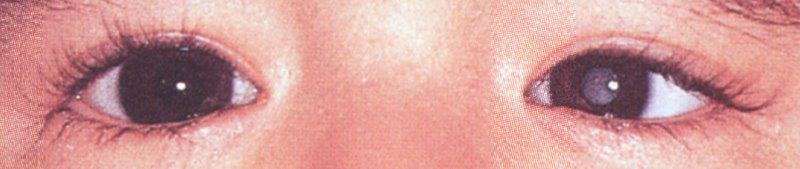
Fig 1. Abnormal red reflex. Credit: Dr Justin Mora
Clinical presentation of congenital cataract
Congenital cataract affects approximately 2.2 per 10,000 births and causes 6.1% of low vision and 4.1% of blindness in children in New Zealand1,2. Presentations vary but the national red reflex screening checks at birth and at six weeks are the optimal times to identify congenital cataract. Early identification and surgical management are key in preventing dense and irreversible sensory deprivation amblyopia. Ideally, for dense unilateral cataracts, surgery and optical correction are performed between six and eight weeks of life and for bilateral cataracts between six and 10 weeks of life3. Surgery earlier than this is associated with an unacceptable glaucoma risk, which nevertheless remains at around 10-25% lifelong.
For the majority of babies, our practice in New Zealand is to avoid intraocular lenses under one year of age and often under two years of age as the evidence shows equal visual outcomes with less complications4. There is no protective effect from intraocular lens implantation on glaucoma risk. Post-operatively babies are optically managed with extended-wear contact lenses, with parents taught how to remove and clean them once a week (Fig 2).
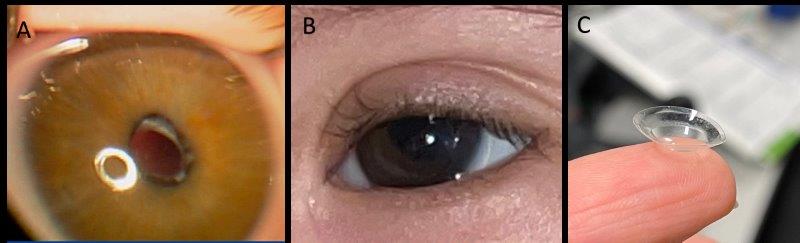
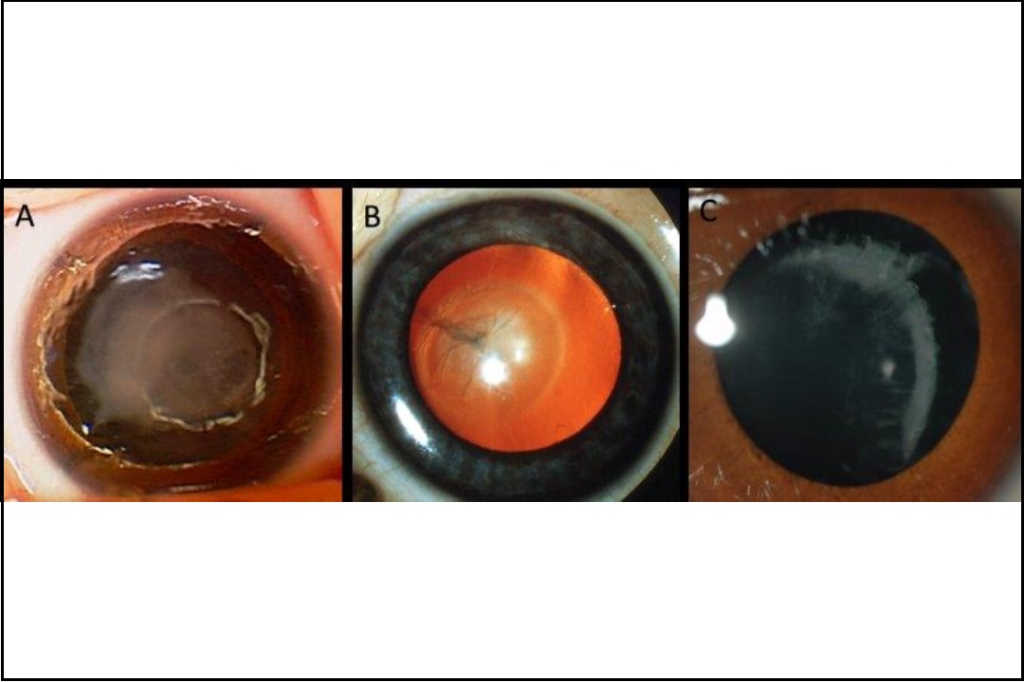
Fig 2. Post-operative imaging in congenital cataract: A) Aphakic appearance with anterior
lens capsule visible just inside the pupil B) Aphakic contact lens (+25) appearance in a baby with trisomy 21 one week after lensectomy, central large button is visible C) Same lens once removed to demonstrate central large button
Investigation of congenital cataract
Half of congenital cataracts are thought to be inherited, with non-genetic causes including intrauterine infection, trauma and developmental anomalies such as persistent foetal vasculature5. The inherited forms encompass not only isolated lens protein genes but metabolic genes, for instance GALAC1 associated with galactosemia and syndromic conditions such as trisomy 21 (Down syndrome, see Fig 2 and Fig 3).
Traditionally, the investigation of congenital cataract involved lengthy and expensive investigations including serum glucose, urinary-reducing substances and other biochemical and metabolic blood tests. However, the approach has changed in the last few years with the advent of accurate and fast NGS gene panels. These can solve up to 75% of bilateral congenital cataract and can be faster than traditional systemic investigations6. One study found traditional investigations yielded a diagnosis in only one of 27 patients with bilateral congenital cataract versus nine of 15 of the same patients undergoing next-generation sequencing testing7.
The latest high-throughput NGS technology permits the parallel sequencing of multiple genes with targeted regions (typically the protein-coding exonic regions plus known intronic variants) read multiple times to ensure accurate and thorough investigation. Complex bioinformatics help to interpret the resulting huge data and filter out likely pathogenic variants (mutations). Panels are flexible and can be updated as new genes are discovered. Identified variants can then be confirmed with Sanger polymerase chain reaction (PCR) sequencing, the gold standard traditional method of sequencing DNA. While the underlying cause of the cataract may be apparent from other ocular findings or known systemic diagnoses, such as trisomy 21, for the remainder, further investigations are warranted to understand systemic risks and the chance of further affected children. NGS gene panel testing of all known genes associated with ophthalmic disease is a broad, accurate and relatively fast approach for these patients6. Our practice in Auckland has changed in recent years to initiate this testing at the time of surgery for patients with suspected congenital cataract with a blood sample taken while under general anaesthesia for DNA extraction. The following two case examples demonstrate how this useful approach can benefit our patients.
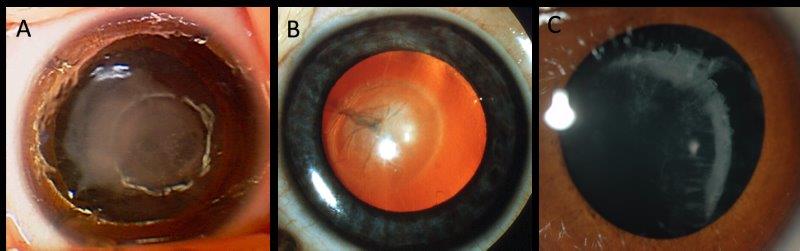
Fig 3. Examples of inherited cataract: A) dense unilateral congenital cataract at birth in a baby with trisomy 21; B) persistent foetal vasculature with posterior lens opacity (Credit: Dr Justin Mora); C) cortical cataract and lens subluxation in a child with Knobloch
syndrome secondary to a homozygous COL18A1 variant
Case 1
A 14-week-old baby failed a red reflex test at the GP and was urgently referred to the eye clinic. Bilateral, asymmetrical cataract was identified. There was no family history of cataract in childhood and no consanguinity. Surgery was delayed due to concurrent sickness. Left lens extraction and anterior vitrectomy were performed at 18 weeks of age with extended-wear contact lens introduced one week later. Blood tests at the time of surgery were sent for an NGS gene panel of 936 genes (Molecular Vision Lab (MVL) Vision Panel v7, Oregon, USA) with results available seven weeks later. This identified a mutation in CRYAA that was previously described in a family with autosomal dominant cataract8.
CRYAA encodes alpha-A crystallin, one of the major proteins that make up the lens. Mutations are thought to cause protein aggregation9. Examination of both parents and the older sibling found no cataract and segregation is underway to prove that this mutation was de novo. The genetic diagnosis provided reassurance that this was an isolated congenital cataract and further systemic investigation was not required.
Case 2
A five-week-old baby was referred to the ophthalmology service with recently noticed anisocoria. Bilateral, asymmetrical cataract were identified with relative right microphthalmia. There was no family history of congenital cataract. The patient was known to have congenital heart disease. Bilateral sequential lens extraction and anterior vitrectomy were performed under the same general anaesthetic at eight weeks of age with contact lens wear started one week later. MVL vision panel v10 (942 genes) was initiated with results available seven weeks later. This found a nonsense mutation in BCOR which encodes BCL6 corepressor that may influence apoptosis and is widely expressed throughout the body10. Mutations in females are associated with X-linked dominant BCOR-related syndrome that has variable systemic involvement including ocular, dental, hearing, skeletal, facial and cardiac. This molecular diagnosis enabled targeted systemic investigation for the patient and explained the congenital heart problems.
Conclusion
These cases illustrate how from one blood test, an accurate and relevant diagnosis was rapidly achieved. For those patients presenting with such a visually significant condition, this change in practice to pursue genetic diagnosis is of great benefit. In the majority of inherited cataracts, gene panel testing is expected to identify the underlying diagnosis.
References
- Wirth MG, Russell-Eggitt IM, Craig JE, Elder JE, Mackey DA. Aetiology of congenital and paediatric cataract in an Australian population. Br J Ophthalmol. Jul 2002;86(7):782-6. doi:10.1136/bjo.86.7.782
- Chong C, McGhee CNJ, Dai SH. Causes of childhood low vision and blindness in New Zealand. Clin Exp Ophthalmol. 03 2019;47(2):165-170. doi:10.1111/ceo.13443
- Self JE, Taylor R, Solebo AL, et al. Cataract management in children: a review of the literature and current practice across five large UK centres. Eye (Lond). 12 2020;34(12):2197-2218. doi:10.1038/s41433-020-1115-6
- Solebo AL, Cumberland P, Rahi JS, Group BICCI. 5-year outcomes after primary intraocular lens implantation in children aged 2 years or younger with congenital or infantile cataract: findings from the IoLunder2 prospective inception cohort study. Lancet Child Adolesc Health. 12 2018;2(12):863-871. doi:10.1016/S2352-4642(18)30317-1
- Francis PJ, Moore AT. Genetics of childhood cataract. Curr Opin Ophthalmol. Feb 2004;15(1):10-5. doi:10.1097/00055735-200402000-00003
- Gillespie RL, O'Sullivan J, Ashworth J, et al. Personalized diagnosis and management of congenital cataract by next-generation sequencing. Ophthalmology. Nov 2014;121(11):2124-37.e1-2. doi:10.1016/j.ophtha.2014.06.006
- Musleh M, Hall G, Lloyd IC, et al. Diagnosing the cause of bilateral paediatric cataracts: comparison of standard testing with a next-generation sequencing approach. Eye (Lond). Sep 2016;30(9):1175-81. doi:10.1038/eye.2016.105
- Yang Z, Su D, Li Q, et al. A R54L mutation of CRYAA associated with autosomal dominant nuclear cataracts in a Chinese family. Curr Eye Res. Dec 2013;38(12):1221-8. doi:10.3109/02713683.2013.811260
- Raju I, Abraham EC. Congenital cataract causing mutants of αA-crystallin/sHSP form aggregates and aggresomes degraded through ubiquitin-proteasome pathway. PLoS One. 2011;6(11):e28085. doi:10.1371/journal.pone.0028085
- Redwood A, Douzgou S, Waller S, et al. Congenital cataracts in females caused by BCOR mutations; report of six further families demonstrating clinical variability and diverse genetic mechanisms. Eur J Med Genet. Feb 2020;63(2):103658. doi:10.1016/j.ejmg.2019.04.015

Dr Sarah Hull completed her medical training at the University of Cambridge and Imperial College, London, and her PhD in paediatric retinal genetics at UCL and Moorfields in 2016. Having completed her ophthalmology training in the UK, she undertook a paediatric fellowship in Auckland and now specialises in paediatrics, strabismus and genetics. She consults in Auckland and at Auckland Eye, and is a senior lecturer at Auckland University.