Implant records IOP for a decade
A German study has found that an implanted telemetric intraocular pressure (IOP) sensor continued to function and was well tolerated in situ for almost 10 years.
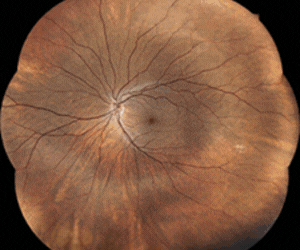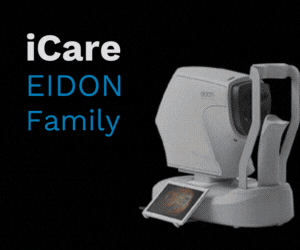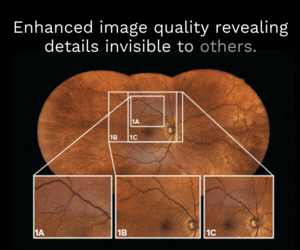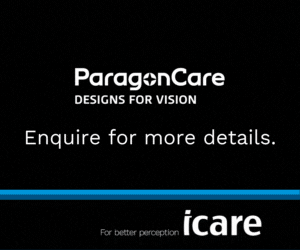
As part of the ARGOS-01 study, researchers led by Dr Ilka Schmidt, University Hospital Aachen, implanted six open-angle glaucoma patients with Implandata Ophthalmic Products’ Eyemate-SC during cataract surgery. Researchers reported that during the 10-year follow-up almost 25,000 IOP measurements were performed, patients had excellent tolerance of the implanted sensor and did not experience sensor-related discomfort or complications.
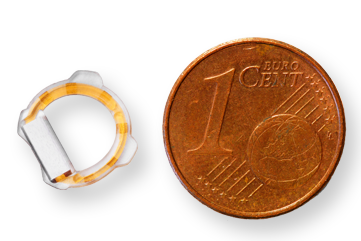
Eyemate-SC works with a hand-held reader that records IOP when it’s held briefly in front of the patient’s eye. After receiving the measurement and saving the data, both reader and implanted sensor are inactive until the next measurement is taken. The system was granted US Food and Drug Administration Breakthrough Device Designation in April 2021 and obtained CE certification two months later.