NZ eye drugs: navigating from drug discovery to FDA approval
The University of Auckland (UoA) has a substantial track record in eye research and is behind a number of important advances in ocular health provision. The Auckland Cataract Study produced reduced risk strategies for phacoemulsification and the Vision Bus Aotearoa, launched in June 2022, provides eyecare services to people in need while raising awareness of eye health. The university spinout Toku Eyes has launched eye screening software for diabetic patients to check their eyes and receive instant results. Auckland-based CatTrax uses cloud-based technologies and expert domain knowledge to provide innovative solutions for the delivery of quality ophthalmic care and the MiSight contact lens is the first FDA-approved therapy to slow the progression of myopia in children.
Another area of marked success has been drug development: a task more challenging in many aspects as it involves complex and highly regulated development pathways and requires significant investment funding (Fig 1). On average, pharmaceuticals take 10-15 years to reach the patient. Following initial discovery, intellectual property needs to be protected, most commonly through patent applications. These start off as a provisional patent, move to the Patent Cooperation Treaty stage and then to the National Phase, all attracting significant legal and patent-office fees in each country for which protection is desired. Without intellectual property protection, investors will not cover the expensive process of drug development, since a third party could produce a generic copy without facing the original developments costs.
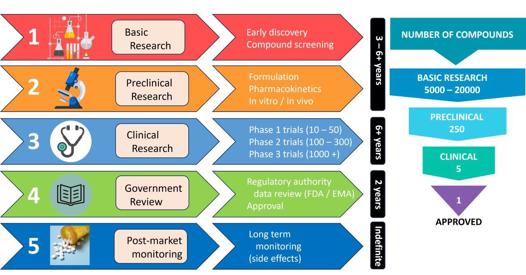
Fig 1. The drug development timeline
After discovery comes a preclinical research phase that includes formulation of the drug into a suitable delivery vehicle, pharmacokinetic studies, stability testing, in vitro and in vivo dosing and efficacy studies, as well as toxicity studies to ensure the formulation is safe. There are then three (sometimes four) levels of in-human clinical trials. The first is a small safety trial to ensure there are no serious adverse effects, the second is to establish that the drug really works for the disease or condition indicated in a carefully selected patient population and the third is a much larger, usually multinational, study for efficacy in the wider population. Then comes a regulatory phase wherein the data is examined – for example, by the Food and Drug Administration (FDA) in the USA, or the European Medicines Agency (EMA) – prior to being approved for use. Along the way, there is significant documentation and record keeping to be filed and updated annually with the regulatory bodies, drug manufacture to clinically acceptable standards, ethics approvals and patients’ informed consent. Financial advisory company Deloitte reports that the top 20 biopharmaceutical companies now spend an average of US$2.3 billion (NZ$3.8bn) to take a new drug from discovery to market1.
From Aotearoa to the world
Despite these challenges, New Zealand punches well above its weight, with three UoA ocular pharmaceuticals concurrently in human trials (Fig 2), two of them at late stage. These are based on research demonstrating the body’s innate immune system inflammasome pathway, first described as recently as 2002, is perpetuated by the opening of a large, non-specific cell membrane channel (termed a connexin hemichannel). Novel compounds developed by UoA researchers regulate that channel’s opening. The inflammasome underlies virtually all types of chronic disease progression, including diseases of the eye such as diabetic retinopathy (DR), age-related macular degeneration (AMD) and chronic (non-healing) injuries.
Nexagon (lufepirsen) ophthalmic gel is an unmodified antisense oligonucleotide that restores the ocular surface by downregulating the pathogenically elevated connexin-43 protein and thus the hemichannel it forms, quenching unregulated inflammation associated with persistent corneal epithelial defects (PCEDs)2. It has a development history of over 20 years, first with the UoA spinout CoDa Therapeutics (NZ) and subsequently with CoDa Therapeutics (USA) Inc. CoDa conducted the first phase 1 clinical trial of Nexagon for the treatment of photorefractive keratectomy and explored the drug’s potential as a topical treatment for non-healing venous and diabetic leg ulcers, until the company ran into financing difficulties and was restructured. The newly formed OcuNexus Therapeutics licensed Nexagon to Eyevance Pharmaceuticals, which formed Amber Ophthalmics, a US based biotech company focused on novel therapies to treat anterior segment disease. Amber’s recent phase 2b multicentre clinical trial evaluated the use of Nexagon in patients with PCEDs due to chemical or thermal injuries. Of those subjects receiving Nexagon, 66.7% achieved complete corneal epithelial recovery, compared to 27.3% of those who received vehicle – a clinically meaningful comparative improvement of 39.4%3. Importantly, those results were achieved in a severe PCED chemical and/or thermal ocular injury population with just three drug administrations over a 28-day treatment period. Nexagon is currently being evaluated for the treatment of PCED due to various underlying causes in a phase 3 study across more than 30 international trial sites.
Xiflam is an oral tablet that closes the pathogenic channel that causes inflammasome-mediated chronic disease progression. It started life as a small molecule with SmithKline (later GlaxoSmithKline) and Proximagen, before UoA research revealed its correct mode of action4. Xiflam is the world’s most advanced inflammasome-regulating drug and is currently in a phase 2b clinical stage trial with InflammX Therapeutics, which specialises in treating autoinflammation due to dysregulation of the innate immune system. Xiflam modifies the disease process without suppressing overall immunity. This current trial, which started in the first quarter of 2023, is for the treatment of diabetic macular oedema. Of the 130 patients enrolled, half were given Xiflam and the other half placebo. The trial is funded and run by the Diabetic Retinopathy Clinical Research network (now renamed DRCR Retina Network) within the National Eye Institute of the USA National Institutes of Health (NIH), representing a significant tick of approval for UoA science. Since DR is associated with chronic kidney disease, the trial is also assessing serum markers for diabetic nephropathy, with support from the Juvenile Diabetes Research Foundation within NIH. InflammX’s second trial will target intermediate AMD, for which there is currently no approved treatment. Intermediate AMD is the dry form of AMD, preceding vision loss from geographic atrophy and wet AMD, and represents 80% of AMD patients. That trial, expected to start in the second quarter of 2024, will enrol 300 patients in three equal groups covering two dose levels and a placebo control. Current treatments for later stage wet AMD require frequent intravitreal injections, which carry the risk of infection, inflammation and discomfort. The intravitreal injection market currently generates over U$S12 billion (NZ$19.7bn) annually, so the potential for an oral tablet to ameliorate AMD progression is considerable.
The third ocular drug is Pachymatrix, being brought into a proof-of-concept trial by UoA spinout TheiaNova, founded in 2022. TheiaNova is funded by Bridgewest Ventures, Auckland UniServices and Callaghan Innovation’s technology incubator programme, and is targeting keratoconus. New Zealand has one of the highest rates of keratoconus in the world, with individuals of Māori and Pacific Island descent disproportionately affected. TheiaNova is developing an early-stage drug combination of the human growth factor TGF-β3 and a low-dose steroid, dexamethasone, as an eye drop that can be administered for periods of up to six weeks to patients with progressive keratoconus. The drug induces cells in the stroma to transiently switch to a chondrogenic-like phenotype that secretes new collagen, thereby strengthening the corneal tissue5. Keratoconus, as an orphan drug (small market size) indication, is the initial target (to both halt disease progression and correct vision), with myopia to follow as a second target, as a similar strategy of corneal reshaping could be employed.
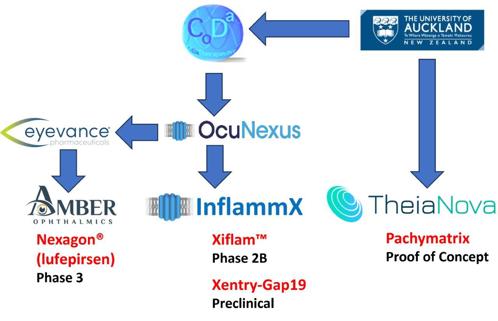
Fig 2. The University of Auckland’s spinout companies and their products
Despite the challenges of time, cost and relatively low success rate outlined in Fig 1, UoA’s Department of Ophthalmology’s foray into drug development, working with the School of Optometry and Vision Sciences, has the potential to make a very positive impact on eye disease treatments. The drugs being developed by InflammX have been used in studies by a joint Johns Hopkins, Harvard and Rutgers’ team as a potential treatment for amyotrophic lateral sclerosis6 and in UoA’s own collaborative studies with the University of Melbourne for multiple sclerosis and brain glioma. These are just the tip of the iceberg for Xiflam applications in central nervous system chronic disease indications, including Alzheimer’s and Parkinson’s disease. With new breakthroughs on the role of the inflammasome in ageing and senescence, the UoA's Department of Ophthalmology has become a centre of excellence for central nervous system research, in relation to both the brain and the eye.
References
- https://www2.deloitte.com/us/en/pages/about-deloitte/articles/press-releases/deloittes-thirteenth-annual-pharmaceutical-innovation-report-pharma-r-and-d-return-on-investment-falls-in-post-pandemic-market.html
- Grupcheva C, Laux W et al. Improved corneal wound healing through modulation of gap junction communication using Connexin43-specific antisense oligodeoxynucleotides. IOVS. 2012;53:1130-1138.
- Scranton S, Hou J et al. A phase 2, randomized, prospective, double-masked, vehicle-controlled study to assess the safety and efficacy of topical NEXAGON in subjects with persistent corneal epithelial defects (PCED) resulting from severe ocular chemical and/or thermal injuries. IOVS June 2023, Vol.64, 3119.
- Kim Y, Griffin J et al. Tonabersat prevents inflammatory damage in the central nervous system by blocking Connexin43 hemichannels. Neurother. 2017; 14:1148-1165.
- Greene C, Green C, Dickinson M, Johnson V, Sherwin T. Keratocytes are induced to produce collagen type II: A new strategy for in vivo corneal matrix regeneration. Exp Cell Res. 2016; 347:241-249.
- Almad A, Taga A et al. Cx43 hemichannels contribute to astrocyte-mediated toxicity in sporadic and familial ALS. PNAS. 2022;119(13):e2107391119.

The University of Auckland’s Professor Emeritus Colin Green is the W&B Hadden chair of ophthalmology and translational vision research. He has co-founded five biotechnology companies and is an inventor on over 465 patents in 24 patent classes. He is the founding scientist and chairman of CoDa Therapeutics NZ, founding scientist and board member of TheiaNova, chief scientific officer at both OcuNexus and InflammX and a consultant for Amber Ophthalmics.